-
-
November 20, 2024 at 6:34 am
naveedullah
SubscriberHello.
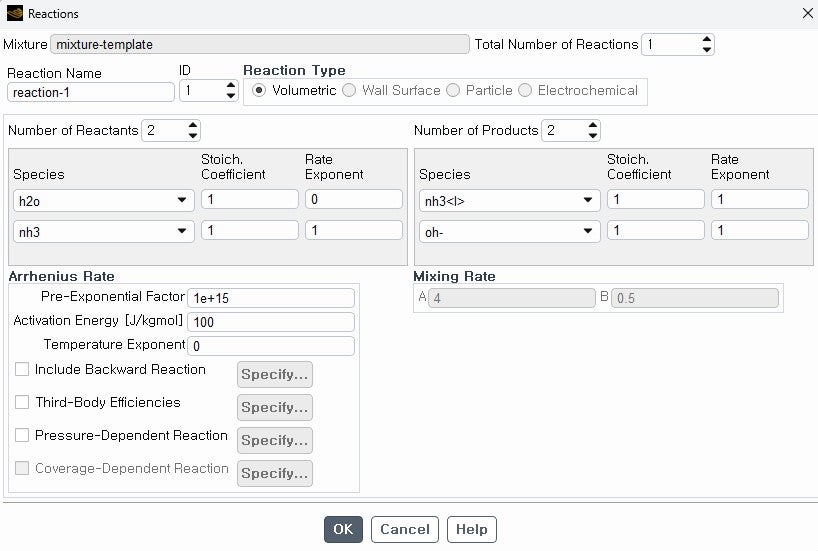
I am using volumetric reactions in my case (ammonia absorption in water) in ANSYS FLUENT. I configured the reaction but am now encountering a warning "
Warning: mass imbalance in reaction-1 stoichiometry. The fluent setup disappears when I run the simulation (shuts down). Can someone please assist me in resolving this problem?
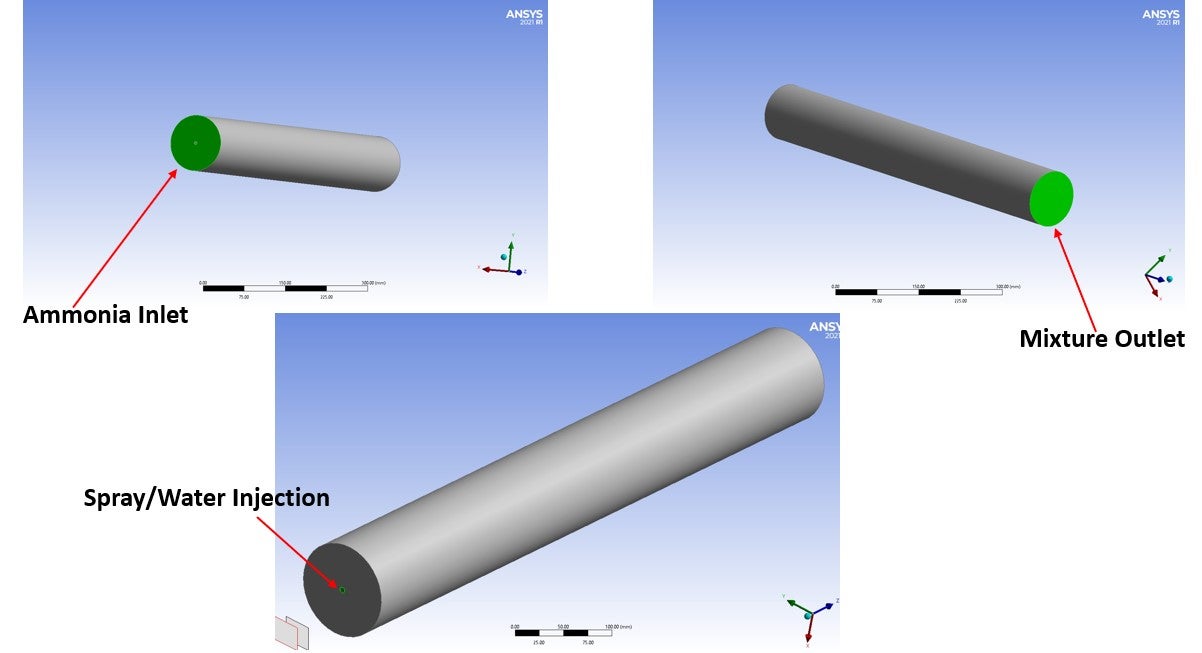
Please look at the attached picture for further clarification.
-
November 20, 2024 at 10:06 am
Rob
Forum ModeratorWhere is the missing mass in the products? Ie where did the H+ go?
-
November 21, 2024 at 6:11 am
naveedullah
SubscriberThank you, Rob
Actually, I am inputting ammonia at the inlet using Eulerian and then injecting water using DPM injection. These two are the reactants. The products are NH4+ and OH- ions. Here in the snapshot, product 1 (nh3l>) properties are modified for the ammonium ion, so no H+ is there. Still, the error, as mentioned above, is there.
Also, could you suggest using the volumetric reaction in species transport (the one I am using) or defining the UDF for water ammonia absorption?
Thank you
-
November 21, 2024 at 12:06 pm
Rob
Forum ModeratorEulerian multiphase and DPM or single phase and DPM?
-
November 21, 2024 at 2:43 pm
naveedullah
SubscriberEulerian multiphase and DPM.
-
November 21, 2024 at 3:47 pm
Rob
Forum ModeratorWhy?
-
November 22, 2024 at 12:25 am
naveedullah
SubscriberMultiphase because I have Ammonia Gas and Water Droplets.
Eulerian is used for ammonia gas and DPM is used for water injection.
-
November 22, 2024 at 11:17 am
Rob
Forum ModeratorIf you have gas and droplets use DPM OR Eulerian Multiphase, not both. Note, Eulerian Multiphase is a model in Fluent, and not to be confused with Euler flow (single phase) as opposed to Lagrangian for DPM tracking.
-
November 24, 2024 at 9:35 am
naveedullah
SubscriberThank you for the reply. So if I use multiphase eulerian with DDPM, then how should I set the water injection? Or it will assign the 2nd phase as disperse phase?
-
November 26, 2024 at 2:00 pm
Rob
Forum ModeratorDo you have sufficient volume fraction to need DDPM? What is the composition of the gas phase?
-
November 28, 2024 at 8:30 am
naveedullah
SubscriberAt the inlet of the pipe, the ammonia volume fraction is 1. The reason for using DDPM is its prompting property for injection. I do not know the reason but using Eulerian without DDPM (using a separate DPM model only), somehow can not see changes in Ammonia volume fraction.
Can you please tell me about the compatibility of using these two models with Species transport for incorporating chemical reactions between water and ammonia?
-
November 28, 2024 at 10:41 am
Rob
Forum ModeratorIf the inlet is all ammonia is that gas or liquid? Where does any other material come from if it's pure ammonia or spray? Please post an image of what you're doing and write on the boundary conditions.
-
November 29, 2024 at 12:42 am
-
November 29, 2024 at 11:11 am
Rob
Forum ModeratorAnd what is the spray volume fraction? Work out what you actually need to model, you've asked about ammonia, NH4+, OH- and so on; so far I don't see much benefit to modelling any reactions if the spray is through a pure species domain.
-
November 29, 2024 at 2:57 pm
naveedullah
Subscriber100 percent water spray through nozzle. So how the mass transfer between ammonia and water take place? Just by physical interaction not chemical reaction?
-
November 29, 2024 at 3:03 pm
Rob
Forum Moderator100% through the nozzle, yes, that's sensible. Now how does that relate to the local cell volume fraction, and therefore whether DPM is an appropriate choice? What happens when the spray hits the wall.
Mass transfer can occur using UDFs, and potentially using surface reaction on the particles if you use DPM.
-
- You must be logged in to reply to this topic.



-
2979
-
970
-
857
-
750
-
599

© 2025 Copyright ANSYS, Inc. All rights reserved.