-
-
January 3, 2026 at 1:35 am
abtharpe42
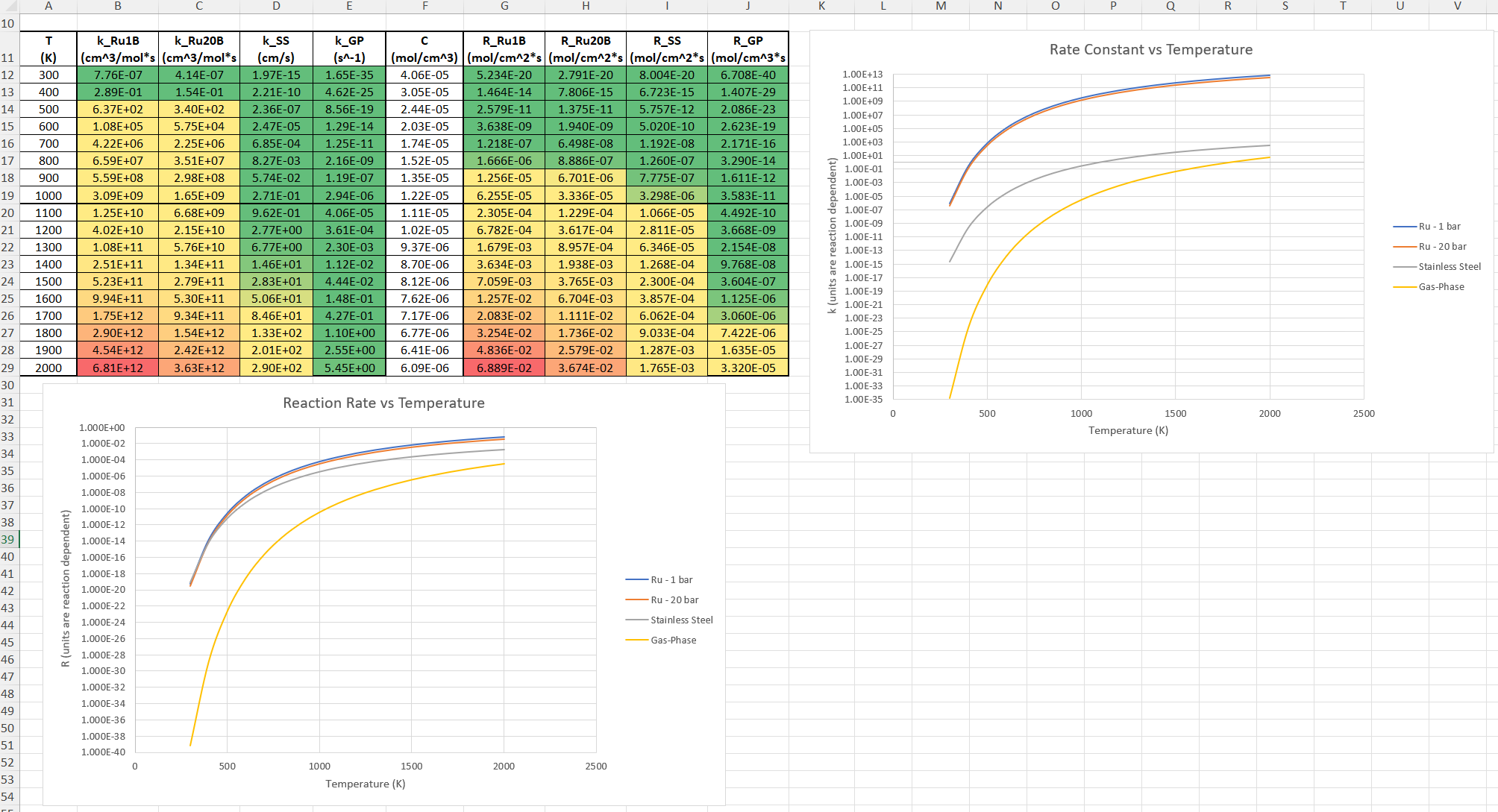
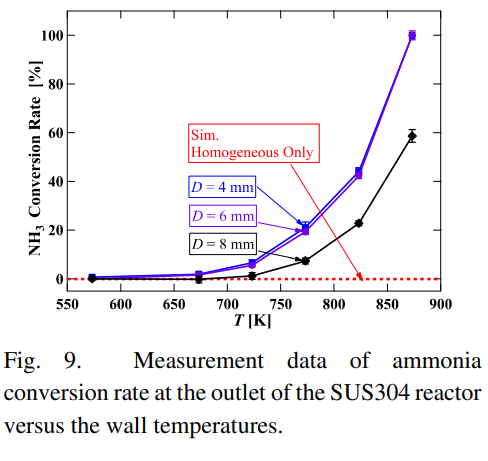
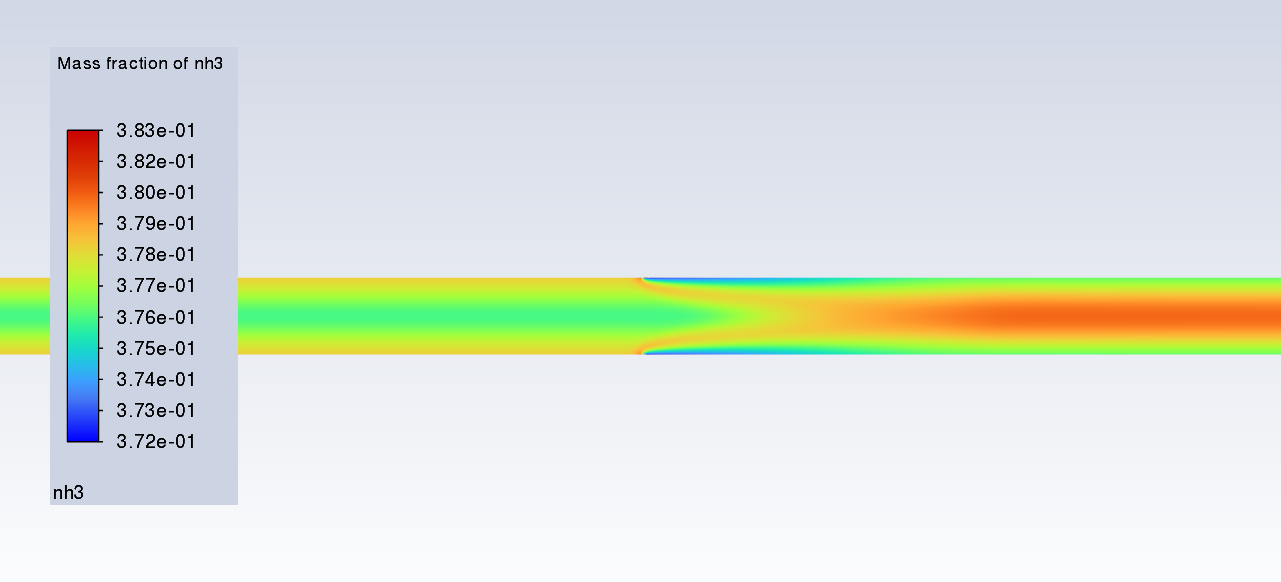
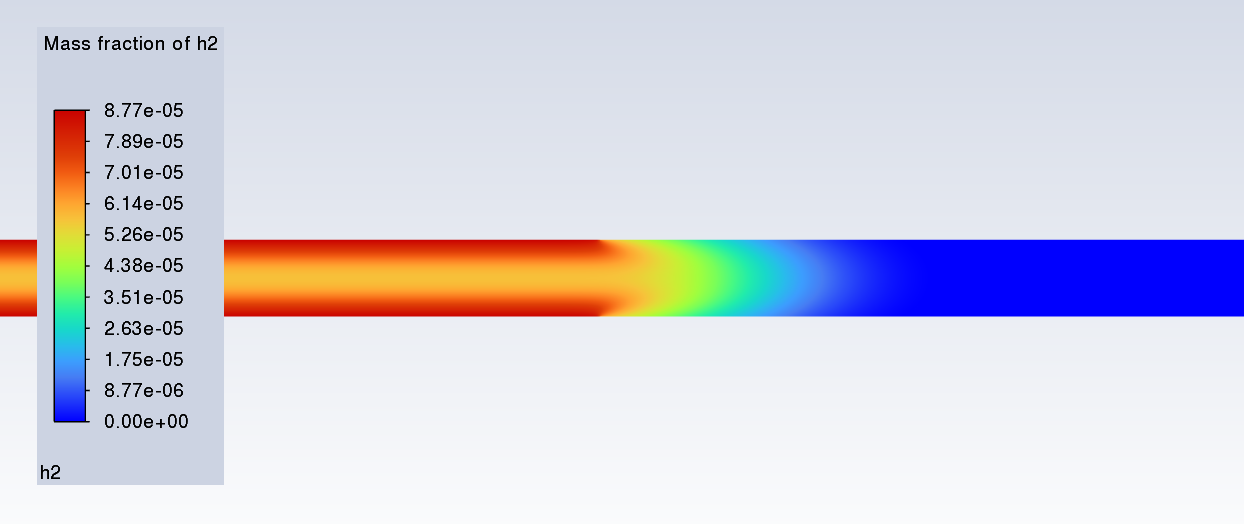
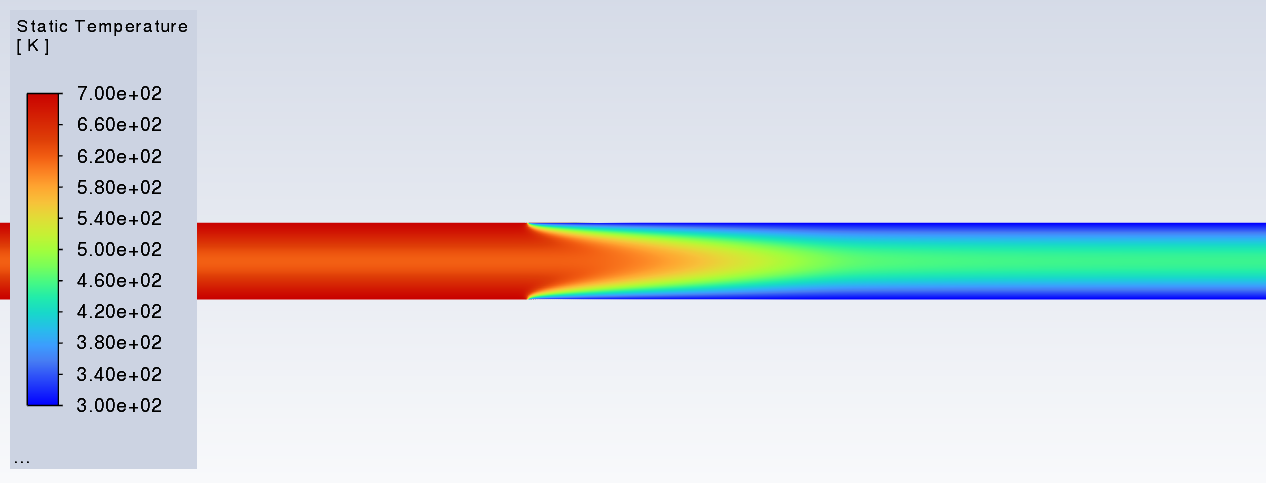
SubscriberI am trying to simulate ammonia decomposition through a channel 4 mm in diameter and 1 m long. The walls of the first 10 cm are set to 300 K, the next 60 cm are set to an elevated temperature that I am varying, and the last 30 cm are also set to 300K. The inlet flow is an ideal gas 1:1 molar mixture of NH3 and N2. The velocity inlet is set to a value such that the average velocity through the hot section is about 7.7 cm/s (what was done in the paper linked later) so the turbulence model was set to laminar. The operating pressure is set to 101325. I have a gas-phase reaction, and I have two sets of surface reactions for different materials (ruthenium for one and stainless steel for the other) to compare against each other. The surface reactions are applied to the hot walls only. The domain is 2D axisymmetric. The gas-phase and stainless steel surface reactions and the channel geometry are from a paper with the DOI: https://doi.org/10.1016/j.ijhydene.2023.04.106 while the ruthenium reaction was given to me by a colleague. The mechanisms are given below where the units for the pre-exponential factor are given in cm, mol, and s where applicable:Gas-Phase Mechanism (r_g=k*C_nh3):ELEMENTSH N NiENDSPECIESH2 N2 NH3ENDREACTIONS KJOULES/MOLENH3 => 1.5H2 + 0.5N2 1.01E7 0.0 240ENDSurface Mechanism for Stainless Steel (r_s=k_s*C_nh3):MATERIAL NI_CATALYSTSITE/Ni_SURFACE/ SDEN/0.1/Ni(S)ENDTHERMO300.0 3000.0 1000.0Ni(S) Ni 1 S 300.0 3000.0 1000.0 10.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 20.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 30.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 4ENDREACTION KJOULES/MOLE MWOFFNH3 => 1.5H2 + 0.5N2 3.1E5 0.0 116ENDSurface Mechanism for Ruthenium (r_s=k_s*C_nh3*C_ru):MATERIAL CATALYSTSITE/Ru_SURFACE/ SDEN/1.66E-9/Ru(S)ENDTHERMORu(S) Ru 1 S 300.0 3000.0 1000.0 10.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 20.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 30.00000000E+00 0.00000000E+00 0.00000000E+00 0.00000000E+00 4ENDREACTIONS KJOULES/MOLE MWOFFNH3 + Ru(S) => 1.5H2 + 0.5N2 + Ru(S) 1.5E16 0.0 128.0ENDHere is a screenshot of my Excel charts showing how rate constant and reaction rate compare with temperature when the pressure is 101325 Pa:A piece of the mesh towards the inlet is shown below:This is the chart from paper that I am trying to follow (they used Cantera for their simulations):The following contours, towards the end of the hot zone, are from a prematurely paused simulation when the hot walls are set to 700 K with the stainless steel catalyst enabled, which should cause a considerable surface reaction rate for both catalysts:As can be seen from the H2 mass fraction and surface reaction results, there is basically no surface reaction happening for either mechanism, even when I go up to 1000 K. For the ruthenium catalyst mechanism, I never had a problem up until this point until fairly recently. All I did recently was clean up the text files to make them easier on the eyes, and this is my first time using the stainless steel mechanism. There are no import errors for the mechanism, and the values of the kinetic parameters as they appear in the Reactions window in are good. Any help I can get would be greatly appreciated.
-
Viewing 0 reply threads
- You must be logged in to reply to this topic.
Innovation Space


Trending discussions


Top Contributors


-
4763
-
1565
-
1386
-
1242
-
1021
Top Rated Tags


© 2026 Copyright ANSYS, Inc. All rights reserved.
Ansys does not support the usage of unauthorized Ansys software. Please visit www.ansys.com to obtain an official distribution.