-
-
April 12, 2024 at 3:16 pm
ghislain_madiot
SubscriberHello,
I am currently work in a project that necessitates the implementation of kinetic mechanisms in ANSYS Fluent. My objective is to refine the reaction mechanism to a simplified one-step reaction model by adjusting the Arrhenius equation parameters. This simplification is crucial for approximating the behavior of a complex system but also for computational cost.
Additionally, I am tasked with modeling an experimental setup that includes a constant UV reactor.
To this end, I have constructed a model with the following characteristics:
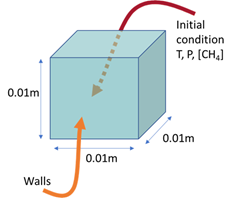
•A cubic volume with a 1 cm³ block.•Boundary conditions define a wall interface.•The system initializes at 2400K.•The absolute pressure is set at 38 Bar.My method involves plotting the mass to verify mass conservation. However, I have observed discrepancies when altering the Arrhenius parameters, particularly changes that affect the mass in the system. These discrepancies are a concern as they indicate potential issues with the reaction rate or mass conservation in the model.
Attached a data sets with varying parameters and the mass evolution over time
Parameters
Sim 1
Sim 2
Sim 3
Sim 4
A
6068000
6068000
6.0 E8
6.0 E8
n
0.1
0.1
2.66e-7
0
Ea
1.825e+08
1.825e+07
1.80 E8
9.125e+07
I would greatly appreciate your insight into the following questions:
1. Are there known issues or typical challenges when adjusting Arrhenius equation parameters in Fluent that could lead to mass discrepancies?2. Could you provide guidance or recommendations on best practices for modifying these parameters to maintain mass conservation?3. Is there any additional documentation or resources that could assist me in troubleshooting and resolving these discrepancies?Thank you for your time and assistance.
Ghislain
-
April 18, 2024 at 8:59 am
Vijay Narayan
Ansys EmployeeHi Ghislian,
There is no known issue with mass conservation.
Reaction Kinetics only dictates the rate of formation of species.
In the plot, the mass corresponds to the total mass within the closed domain ?
I'm assuming you have only 1 cell zone and the mass is reported as Volume intergral report.
Also, I'm assuming you are running it as Transient and the domain is a premixed system of your reactant species ( I see a mention of CH4, but there is an oxidizer also rt ?). Plus patching the entire domain to high temp of 2400K could trigger the reaction in the complete domain and could pose challenges in the simulation run.
In Summary, I would require details based on my above comments to assist your further.
-
April 18, 2024 at 9:22 am
ghislain_madiot
SubscriberHi Vijay,
Thank you for your reply.
I can confirm your assumptions are correct.
The plotrepresents the total mass within the enclosed domain, and this mass is calculated as a volume integral.
This simulation is transient.
The initial conditions are set with methane (CH4) at 100%, a temperature of 2400K, and a pressure of 39 atm.
The reaction modeled is the one-step thermal decomposition of methane: CH4 ==> 2H2 + C.
Additionally, I have utilized this method for more complex reactions and compared the results with Chemkin, finding them to be similar, without any issues during the simulation runs.
Ghislain
-
May 9, 2024 at 4:40 am
Vijay Narayan
Ansys EmployeeHi Ghislian,
Thanks for sharing the details and sorry for the delay in response. Could you share the below details as well.
a) FR/No TCI or ED/FR model and which chemistry solver you are using.
b) Also what is the rate exponent you are using ? For global reactions the rate exponent need not be unity.
c) what is the time step size and solver (SIMPLEC or PISO) you are using ?
-
May 9, 2024 at 5:19 am
Vijay Narayan
Ansys EmployeeOne more thing, what is the density formulation being used. By default it is incompressible ideal gas. Please change it to ideal gas formulation as your closed system pressure is also changing.
Try using a smaller time step of 1e-8 sec - you can increase it later and share your observation
-
May 13, 2024 at 3:05 pm
ghislain_madiot
SubscriberHi Vijay,
Here the information you asked:
- I use Finit Rate / No TCI with the Explicit source for chemistry solver.
- Density formulation in the initial case is ideal gas
- Rate exposure is equal to 1
- The initial time step in the plot were 10e-5 which corresponds to the Chemkin methodology, but I noticed the same behavior with lower time step like 10e-7. I quick run a case with 10e-8 same result.
- Scheme used is Coupled, however I ran the same case with SIMPLEC and PISO and obtained even higher value of mass
I put the screenshots for the differents menu
Perhaps, it might be usefull if I share the model or the setting file with you.
Regards,
Ghislain
-
- The topic ‘Kinetic Mechanisms and Arrhenius Equation Parameters in ANSYS Fluent’ is closed to new replies.



-
3467
-
1057
-
1051
-
929
-
896

© 2025 Copyright ANSYS, Inc. All rights reserved.