-
-
February 7, 2025 at 1:41 pm
tolgak.338
SubscriberHello,
I’m new to chemical flow simulations and currently using ANSYS Fluent 2024 R2 (Student Version) to model CO₂ injection onto a stationary Ca(OH)₂ slurry. The goal is to simulate CO₂ diffusion, dissolution, and reaction leading to CaCO₃ precipitation. Due to hardware limits (4 cores, mesh restrictions), I’m working in 2D.
Key Questions:
- How to define CO₂ diffusion into slurry correctly? Should the slurry initially be modeled as just Ca(OH)₂ + water, with CO₂ diffusing into it over time, or should CO₂ be included in the slurry phase from the start?
- How to define reaction and mass transfer? How should I correctly set up the dissolution of CO₂ into the slurry and its reaction with Ca(OH)₂? Should mass transfer between phases be explicitly defined?
Current Setup:
- Multiphase Model: Eulerian with three phases:
- Gas phase: Initially stationary air; CO₂ injected at 6 mL/s (approx. 45 m/s) from a 0.41 mm nozzle. (Defined as an air-CO₂ mixture since Fluent requires multiple species per phase.)
- Slurry phase: Ca(OH)₂ + water, defined as a fluid with solid properties.
- CaCO₃ solid phase: Defined as granular fluid due to Fluent’s multiphase restrictions.
- Turbulence Model: Realizable k-ε with scalable wall functions.
- Initial Condition: Domain starts filled with stationary air.
Issues & Questions:
- Divergence Problems: The simulation immediately diverges (most probably because of boundary conditions). Any suggestions on stabilizing it?
- Material Definition: Is defining CaCO₃ as a granular fluid the right approach?
- Boundary Condition for Slurry: Should it be stationary (wall or pressure outlet?) or have a small velocity (currently 1e-6 m/s inlet)?
- Chemkin Availability: Chemkin doesn’t appear as an option in Species Transport. Is it available in the Student Version? If not, what alternatives can I use?
- Tracking pH Variations: What’s the best way to compute and visualize pH in Fluent?
- Measuring CO₂ Diffusion Depth: How can I track how deep CO₂ penetrates into the slurry before reacting?
Since I’m still learning how to correctly model multiphase chemical flows, any guidance would be really helpful!
Thanks in advance.
-
February 10, 2025 at 9:56 pm
jcooper
Ansys EmployeeHi:
I would suggest doing a literature survey of both experiments and simulations so that you can better understand the process. This analysis is quite complex and a bit beyond something that a Forum can provide very detailed advice on. There are many configurations of this type of process, and the configuration will dictate the best setup options.
The dissolution/mass transfer of CO2 from the gas phase into the slurry is usually the rate-controlling step. Once the CO2 enters the slurry reaction occurs almost immediately, so the typical setup will inject CO2 above the slurry and then increase pressure and temperature to enhance the mass transfer. (CO2 can also be injected directly into the slurry.)
You should therefore start with the CO2 and Slurry separated, unless the system is one that forces CO2 into the slurry.
The reactions you can setup may limit how the species are defined. The calcium hydroxide reaction can be setup using two species transport mixtures: gaseous species transport mixture (water, air, Co2) and semisolid (CaCo3/CaOH2 slurry with a special version of CO2 to account for the dissolved CO2). You could separate out the CaCO3 eventually, but I would start with the simpler setup as proof of concept. Adding a third phase may greatly decrease the robustness of the analysis.
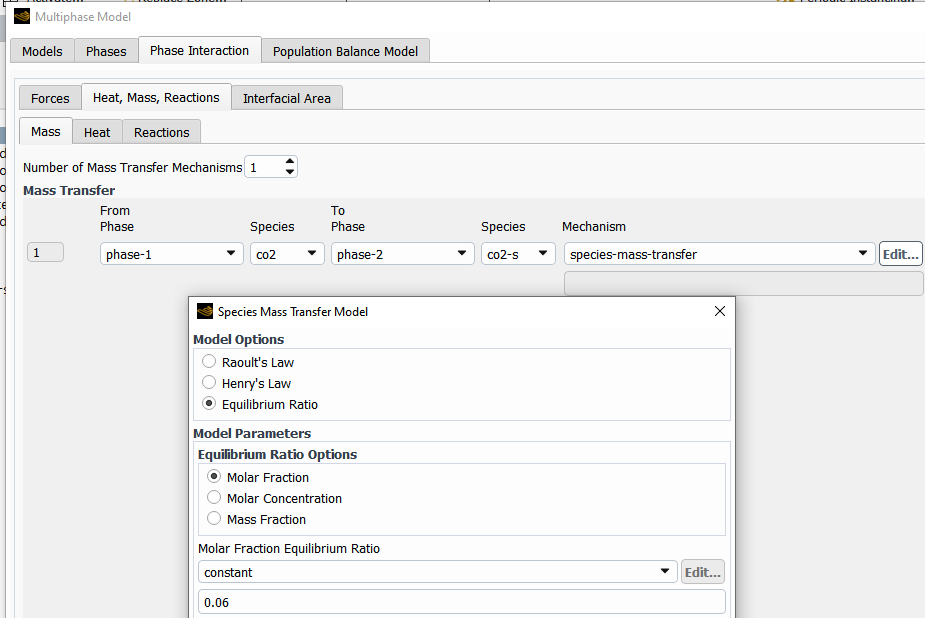
For mass transfer, you can setup an equilibrium ratio to limit the dissolution rate once a certain concentration is reached, (but you may need to experiment with this)
For the reaction:
You will need to find a temperature-dependent rate expression for this reaction in the literature
- Material Definition: Is defining CaCO₃ as a granular fluid the right approach?
This depends on what you are trying to do. The setup above with two phases will not distinguish between the slurry and CaCO3, but it might be more stable. If you need to track the CaCO3, you will need a third phase so that the CaCO3 can ride on its own velocity field.
- Boundary Condition for Slurry: Should it be stationary (wall or pressure outlet?) or have a small velocity (currently 1e-6 m/s inlet)?
The slurry will be a fluid bath or phase in your analysis and should be a volume in your solution fields that is initialized with the initial volume fractions of water and Ca(OH)2. (I am basing this suggestion on your description of injecting CO2 onto/above a slurry.)
- Chemkin Availability: Chemkin doesn’t appear as an option in Species Transport. Is it available in the Student Version? If not, what alternatives can I use?
You won't have access to Chemkin for reactions because your reaction will be heterogeneous. Unless you have a Chemkin mechanism, this doesn't matter though. Fluent can calculate and solve reaction chemistry with its own solver.
- Tracking pH Variations: What’s the best way to compute and visualize pH in Fluent?
You can create a custom field function to calculate pH from existing quantities. Custom field functions can be visualized in the same way as variables
- Measuring CO₂ Diffusion Depth: How can I track how deep CO₂ penetrates into the slurry before reacting?
There will be a separate CO2 variable in the slurry phase that the gas phase CO2 transfers into (as shown above). This variable can be used to show where the CO2 goes. I doubt it will penetrate very deeply, as it will react quite quickly
I hope this helps,
Judy
-
- You must be logged in to reply to this topic.



-
4607
-
1515
-
1386
-
1209
-
1021

© 2025 Copyright ANSYS, Inc. All rights reserved.