TAGGED: define-source
-
-
November 20, 2023 at 2:43 am
Bruce Zhou
SubscriberHello !
I am now working on a problem involving chemical reaction using Fluent 19.2. While Fluent provides the method for modeling chemical reaction problems, I am trying to simulate the problem with Eulerian multiphase model by adding source term using UDF(DEFINE_SOURCE macro). But I have met several problems.
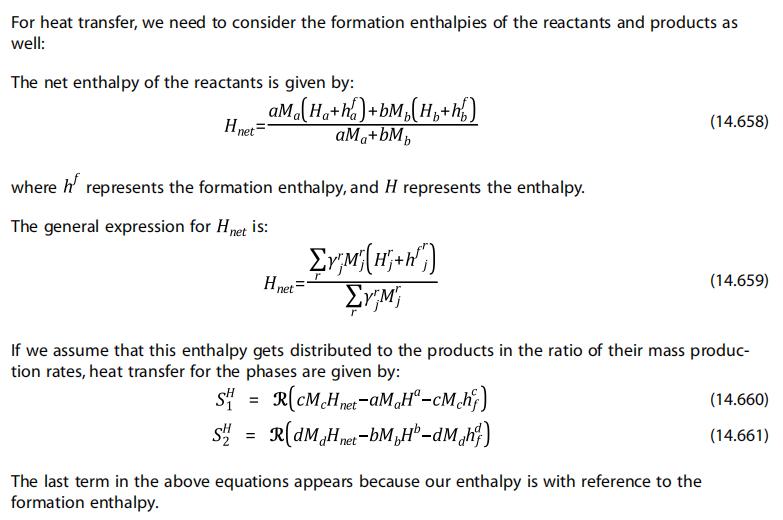
Firstly, I am now referring to Section 14.8.2.1(Source Terms due to Heterogeneous Reactions) of the help document. In the part of heat transfer(14.8.2.1.4, figure shown below), the letter a in Ha term is subscript in Eq. (14.658) while in Eq. (14.660) it is superscript. Is there any difference between the two Ha terms in the two expressions ?
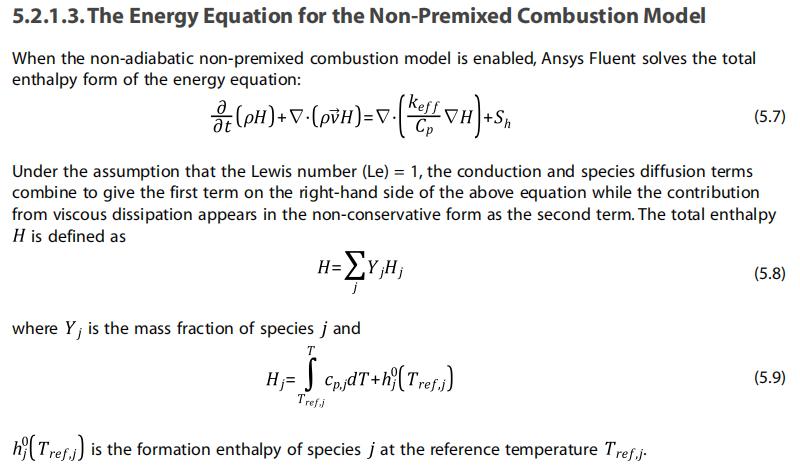
Secondly, I have selected the species transport model(as well as Eulerian multiphase model, 1 mixture phase and 2 pure phases), but not selecting any options related with chemical reaction. According to the help document, when non-premixed combustion model is selected, the energy equation solved is in the form shown in the figure below(Section 5.2.1.3)
Since I did not enable the above-mentioned model, is that mean the energy equation in my problem should be the form shown below(Section 5.2.1.1) ? In the dialogue of material property setting, it is found that the standard state enthalpy and reference temperature could be input, and I am wondering if the input value makes sense in my case.
Thirdly, to add energy source term related with chemical reaction manually using UDF, what is the term for the consumed phase? Is the formation enthalpy of the phase material should be included or just the sensible heat ?
-
November 21, 2023 at 2:28 pm
Rob
Forum ModeratorAny reason for not using the species reactions within a phase, or the chemistry options in the Phase panel in Fluent? You also want to update to 2023R2 (soon to be superceded by 2024R1) for new feature that will help with what you're doing.
If materials are changing phase, you would usually include two materials, with one being solid/liquid and the second liquid/gas respectively. Formation enthalpy then includes the chemical and "phase" part (eg latent heat).
-
November 22, 2023 at 1:10 am
Bruce Zhou
SubscriberThanks for your answer ! The reason I did not use the chemistry options in Fluent is that I want to get a better understanding on defining source with UDF. Since numerical formulation for chemical reaction simulation is provided in detail in the help document (theory guide), I thought this is a good reference. What now I am wondering is: is it possible for simulating a fluid flow problem involving chemical reaction with Eulerian multiphase model + Source UDF?Is there any difference in the simulation results gained through 19.2 and 2023 version? The chemical reaction in my problem is a decomposition reaction (A (liquid)= B(gas) + C(liquid)). "the chemical and phase part(eg latent heat)" what is the chemical part?
-
-
November 22, 2023 at 10:31 am
Rob
Forum ModeratorChemicals have a formation energy, but also energy associated with state. In Fluent these two components are lumped together as enthalpy. That's fine for separate models with reactions OR phase change but can be a little messier when it's AND. So, use some caution.
I assume your reaction is actually A = B + C + energy? The energy term will come from the enthalpy. However, you're also adding and removing mass from the domain against a reference of 298.15K so will need to add and remove heat from the phases.
Do A and C mix? Ie is the liquid a species mixture or two separate phases?
R19.2 is around 5 years old now, and multiphase saw significant improvements in the 2019-2021 period that will make a difference. Changes since are equally useful but may be less obvious.
-
November 22, 2023 at 12:47 pm
Bruce Zhou
SubscriberThanks for the answer! A, B and C are three separate fluid phases that don't mix with each other. A is a three-component mixture with one of the component in chemical reaction while B and C is pure substance. "However, you're also adding and removing mass from the domain against a reference of 298.15K so will need to add and remove heat from the phases. ", so the energy source term for phase A is -mass*(formation enthalpy of the component in reaction+sensible heat) or -mass*(sensible heat)? What is the energy source term for phase B and C?
-
-
November 22, 2023 at 1:21 pm
Rob
Forum ModeratorAs you're using a UDF, you can set what you want. Then you just need to make one phase hotter/colder to suit.
-
- The topic ‘Energy source term related with chemical reaction in UDF’ is closed to new replies.



-
4989
-
1665
-
1386
-
1242
-
1021

© 2026 Copyright ANSYS, Inc. All rights reserved.