TAGGED: #fluent-#ansys, electrochemistry
-
-
January 10, 2023 at 11:16 pm
David_SY
Subscriber -
January 10, 2023 at 11:25 pm
David_SY
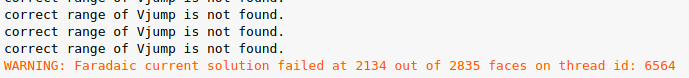
SubscriberI am using "Potential". I got a warning and the simulation diverged:
My model has two electrochemical reactions for electrodes.
I set a certain potential value for each electrode.
I noticed UDSs were automatically loaded, so I disabled them since I did not use UDFs.
Someone said It's hard to converge due to the numerical instability in the range of low concentrations.
Do I have to use UDFs for electrochemical reactions, or is there a way to converge?
Thank you for your help! -
April 27, 2023 at 12:58 pm
Konstantin
Ansys EmployeeHello,
Some models in Fluent, including all thosed which are available as add-on modules, use UDS infrastructure, and as such, preloaded UDS must not be deactivated as they are used in the model.
-
April 27, 2023 at 5:22 pm
David_SY
SubscriberThanks for your input.
We were using 2022R2 and now switched back to 2022R1. Then, we haven't had the warning message.
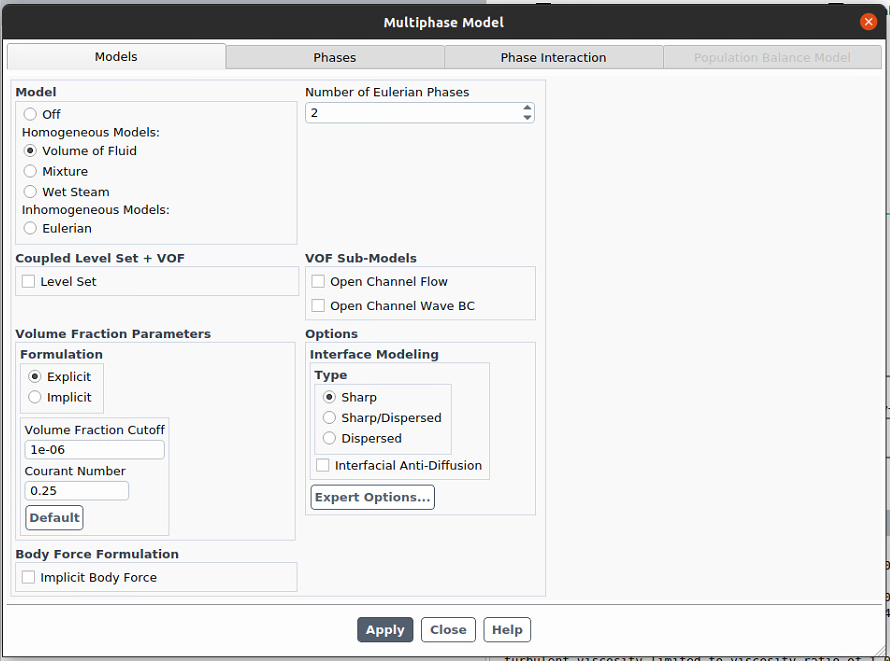
However, we are having a divergence issue with our multiphase geometry. The mesh quality is almost perfect.
We are assuming that the divergence is caused by the multiphase mode because we removed gas phase from our model and ran it with the electrochemical package. It worked well. The new model (without gas phase) is working fine and there is no difference between the old one (multiphase) and the new one (only liquid) other than multiphase.Can you give me advice for this? Please let me know if you need further information of our model
Thank you for your help. -
April 27, 2023 at 6:24 pm
Konstantin
Ansys EmployeeWhich specific electrochemistry model are you using (please share screen shots), as we have several models which are defines as electrochemistry?
-
April 27, 2023 at 6:40 pm
-
April 27, 2023 at 7:12 pm
Konstantin
Ansys EmployeeI see only the potential model been used, without the electrochemistry part. All it does is it solves an extra potential equation which won't have an effect on the fluid flow unless you have Joule heating option on, which you don't. So whatever divergence comes from either the VOF model or species transport. A good first step would be to troubleshoot them one by one: start with VOF model only without surface tension, turn off the poential and species transport, and check if it works. We have a good example illustration a VOF setup on our Ansys Innovation Courses site:
/courses/index.php/courses/fluid-statics/lessons/simulation-examples-homework-and-quizzes-3/topic/simulation-example-u-tube-manometer/
Note that an initial patch of the secondary (gaseous phase in your case) in a region where the seondary phase is first expected to appear is recommended for a better start up of the simulation.
Once the VOF simulation going, add complexity as needed.
-
May 1, 2023 at 5:38 pm
David_SY
SubscriberThank you for all your help.
I deactivated the vapor phase and ran only the liquid phase with the electrochemical mode. It worked fine.
I also ran multiphase without the electrochemical mode, and it also worked fine.
However, once I added the electrochemical mode, it diverged.I am wondering if you have a tutorial of multiphase simulations with the electrochemical mode.
Now that there is lack of information about electrochemical simulations in the Ansys manuals, it is challenging to figure this out.Thank you again.
-
May 2, 2023 at 12:00 am
-
May 2, 2023 at 4:32 am
David_SY
SubscriberSorry for the confusion.
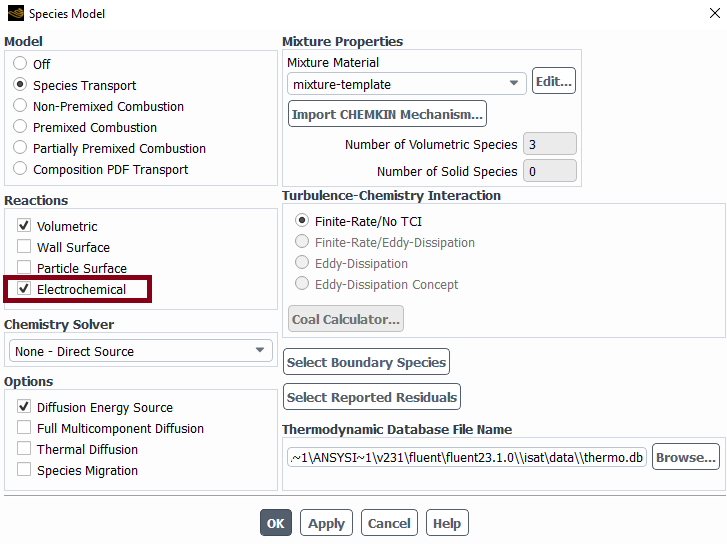
\Yes, we are defining electrochemical reactions in the mixture template as below:
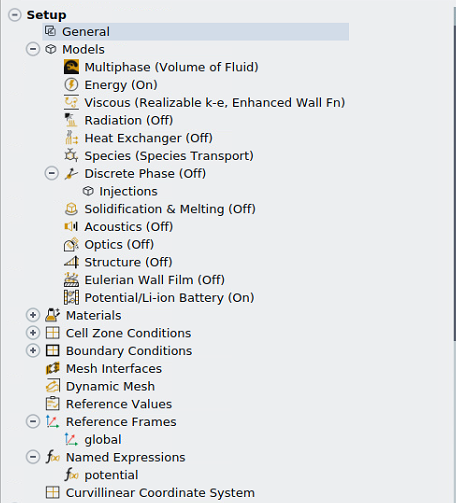
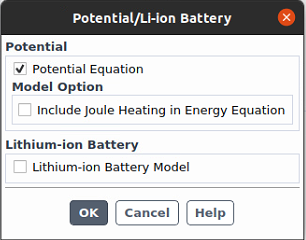
What I mean by "turn off the electrochemical model" is that I deactivate "Electrochemical" reactions under "Species Model" and also turn off "Potential/Li-ion Battery" under "Models". When we deactivated the electrochemical reactions and turned off "Potential/Li-Ion Batter", it worked well.
For your reference, we have two phases (gas, liquid) in a 3D reactor design, and two electrochemical reactions only occur in electrolyte phase (liquid phase). We add a certain negative current density on an electrode where an electrochemical reaction occurs. We set the potential (V) on the other electrode to 0 in which the other electrochemical reaction occurs.
If you need more information, please let me know.
Many thanks. -
May 3, 2023 at 8:36 pm
jcooper
Ansys EmployeeHi David:
Does the divergence for the multiphase case with electrochemistry occur in both 2022R1 and 2022R2?
The error you are getting can occur when the elextrochemical reactions are occurring too quickly or when they are being driven in the wrong direction by external conditions, such as imposed boundary conditions, or divergence. This can happen if the reaction rates are set up incorrectly, or if the timestep is too large. (Timesteps of 1.0e-04 s may be required to start off cases until the species stabilize.)
When the electrochemistry model only operates in one phase, the only interaction between the electrochemistry framework and the gas phase will occur at the phase interface, where heat and possibly momentum transfer occurs. (I am assuming that there is no interphase mass transfer?) The phase boundary can therefore affect the reaction rates if excess heat is removed or transferred.
What is the role of the gas phase in this analysis? Is it transferring heat to or from the liquid (electrolyte) phase? Is the electrolyte phase flowing or stationary? Are your phases setup so that the species are in the solved (primary) phase?
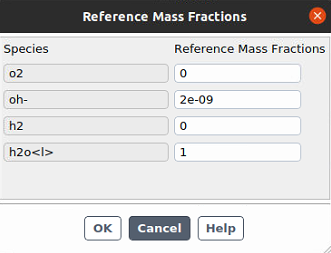
If you can send screenshots of both your reactions and their electrochemical rate setup, this will be helpful. Please note that a reference concentration is required for any specie on which the reaction rate(s) are dependent. Reference species concentrations default to zero, which can lead to infinite or zero reaction rate for rate-determining species.
Best Regards,
Judy
-
May 3, 2023 at 9:27 pm
David_SY
SubscriberHello Judy,
Thank you for your reply.
Please ignore the first reply that I made. We don't have the error message anymore, but just have the divergence issue.We have an inlet in the liquid phase and an outlet in the gas phase. Feed gas (as bubbles) introduces via the inlet, reacts, and generate byproducts.
The byproducts and remaining feed gas are transferred from the liquid phase to the gas phase, and finally go out through the outlet.
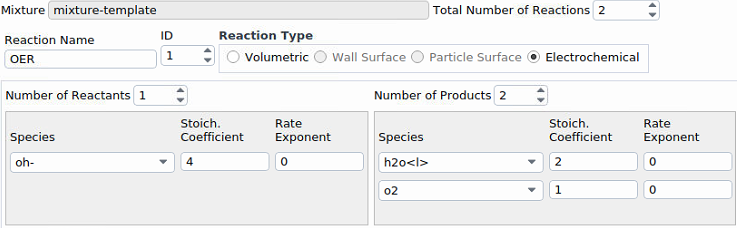
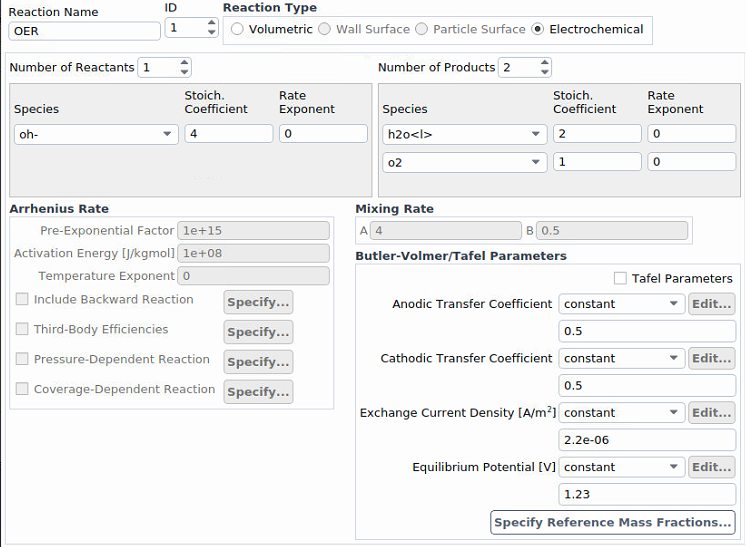
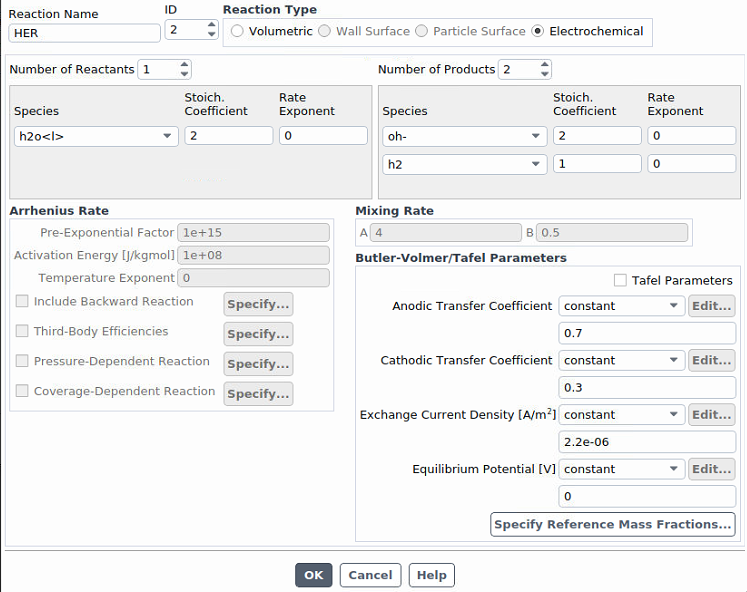
Our electrode rotates (using "moving wall"), and therefore the electrolyte (the liquid phase) is stirred. The temperature is almost maintained constant (room temperature). Initially, the liquid phase contains water with a very low amount of an ion, and the gas phase is nitrogen.For the first step, we switched our inlet and outlet to walls and will switch back later on. Thus, we are starting with simple reactions without inlets and outlet. We will definitely add more reactions. I've attached screenshots. If you need further information, please feel free to ask me.
Thank you for your time and help.
-
May 3, 2023 at 11:07 pm
-
May 4, 2023 at 6:13 pm
jcooper
Ansys EmployeeI would set a small, non-zero number for o2 reference mass fraction because it is a driving specie.
What gases are being bubbled through the liquid? Just o2 or a mixture of h2 and o2? I would also be interested in knowing how you are initializing this case, as all chemistry can be sensitive to initialization. Reactions are expected to go in the forward direction to generate the expected voltage, but they may reverse under some conditions and this will generate nonsensical voltage and divergence. Sometimes it helps to boost the exchange current density to see more quickly what trend things are headed toward, as very low exchange current densities can result in round off error, noisy solution and divergence. (All electrochemistry cases should be solved with double precision.)
I also want to add here that multiphase electrochemistry in Fluent works in theory, but has not been tested extensively, so you may be encountering a modelling limitation. If it works with electrochemistry treated entirely in the liquid phase, but not with both phases, then this is the most likely cause of the divergence.
-
- The topic ‘Potential Model’ is closed to new replies.



-
3044
-
971
-
884
-
858
-
792

© 2025 Copyright ANSYS, Inc. All rights reserved.