-
-
September 27, 2022 at 8:52 am
hbachiri
SubscriberHello,
I am simulating a methanation reaction, where I used a UDF to calculate the reaction rate.
In order to calculate the rate of reaction, I need first to calculate the partial pressures of different species.
Q1/ What is the total pressure ? is it C_P(c,t)? or do I need to add the operating pressure to it?
Q2/ Is there a way to take into consideration the heat of the chemical reaction (shown in equation 5.10 ), other than using UDF?

I would appreciate any help you could provide.
Thank you
-
September 27, 2022 at 3:02 pm
Rob
Forum ModeratorI suspect C_P(c,t) is static pressure, return the value to a UDM to check.
Re the chemical reaction, that may depend on what you're coding up. Are you using a custom reaction or sink/source terms?
-
September 27, 2022 at 3:55 pm
hbachiri
SubscriberThank you Rob, yes I also think it is static pressure.
Yes, I am using a custom reaction. I have calculated the volumetric rate using a UDF and now I would like to add the energy source term using equation 5.10
-
September 27, 2022 at 4:00 pm
Rob
Forum ModeratorIf you've set the species formation enthalpy does that do it for you?
-
October 1, 2022 at 5:04 pm
hbachiri
SubscriberThank you Rob for your response, and sorry for taking long to answer back.
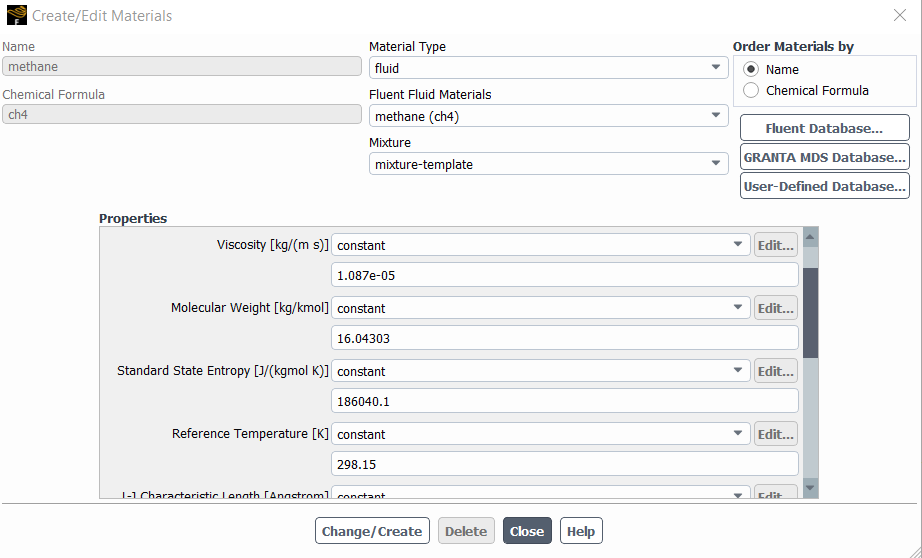
I know that usually, heat of formation is a given property in fluent, though I can not find it as you can see in the following picture.
So I don’t know where I should set the heat of formation?
And whether I need to use a UDF to calculate the heat of reaction or it is calculated automatically? (My objective is not to get the value of the heat of reaction, I only want to represent the energy conservation equation in the reactive zone correctly)
-
October 3, 2022 at 8:28 am
Rob
Forum ModeratorI think it's the difference in the entropy: the reactants and products will sum to a different value, and the difference is heat gain/loss into the system.
With a custom reaction the solver may do that for you.
With source terms you need to work it out yourself.
-
October 7, 2022 at 7:09 pm
hbachiri
SubscriberThank you so much
-
- The topic ‘Methanation reaction in porous zone’ is closed to new replies.



-
4673
-
1565
-
1386
-
1236
-
1021

© 2025 Copyright ANSYS, Inc. All rights reserved.