-
-
March 24, 2022 at 2:36 pm
Yago
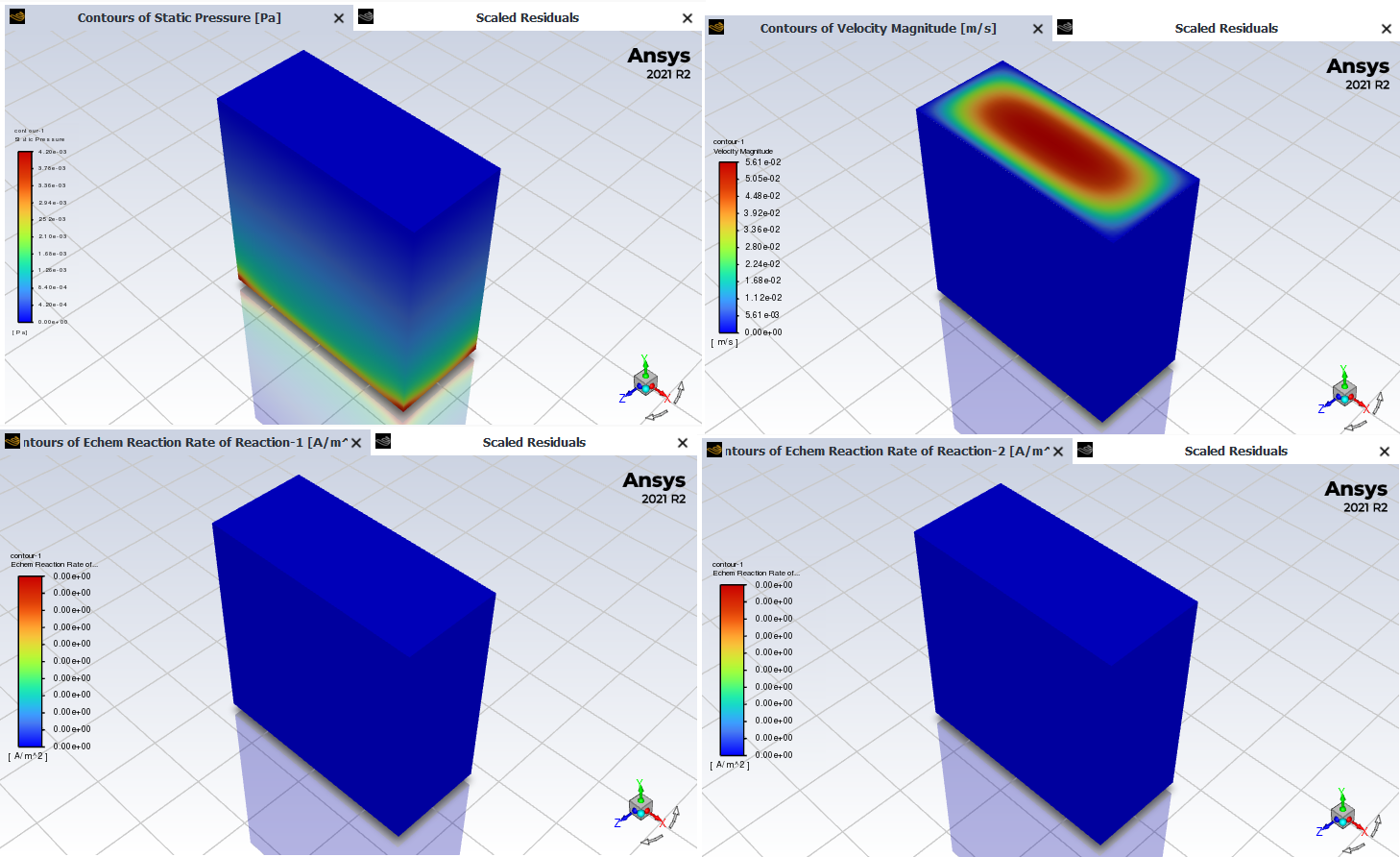
SubscriberI'm trying the simulate a water splitting electrochemical reaction using the "electrochemical reaction" model in Fluent. The geometry is basically a prism, which would be the electrolyte, with an electrode on each side (for cathode and anode). It also has an inlet and outlet in the top and bottom sides. I want to have the anodic reaction (2 H2O -> O2 + 4H+) on the surface with the anode and the cathodic reaction (2 H+ -> H2) on the other surface. I've tried to follow the user's guide and what it says, but once I calculate the simulation, there doesn't appear to be any reaction. The pressure and velocity contour graphics show that there is flow of material, but there's no reaction heat nor reaction rate. I've tried the same model but with the volumetric reaction and everything works, but when I do it with the electrochemical model, there's no reaction.
Maybe someone has had the same problem or knows any common mistake when doing this kind of reaction.
Thanks for your help.
March 30, 2022 at 12:23 pmKarthik Remella
AdministratorHello:
Are you checking both Volumetric and Electrochemical in your species model? If not, could you please give this a try? The electric potential solver will be enabled this way.
Karthik
March 30, 2022 at 12:30 pmYago
SubscriberHello, I am indeed checking both, Volumetric and Electrochemical in the species model.
Thanks for the suggestion though.
April 1, 2022 at 4:19 pmJohn Ibrahim
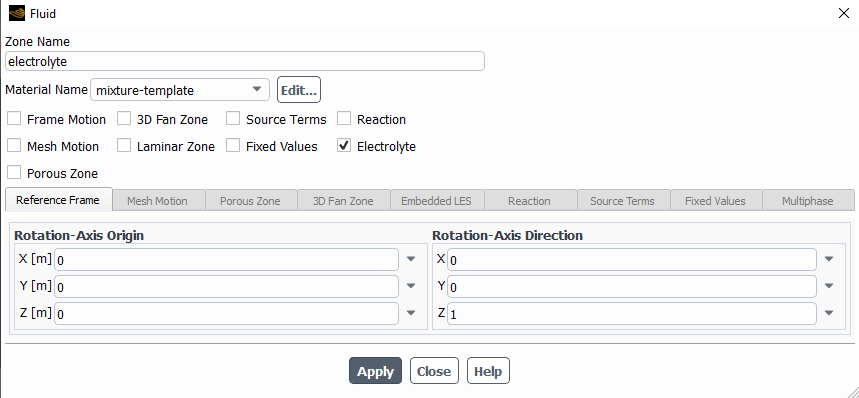
Ansys EmployeeHello 1-Make sure you have assigned the reaction as electrochenistry reaction.
2-material properties->mechanism. Make sure the mechanism I is selcted.
3-Make sure you have enabled the reaction on a wall.
4-Make sure you have assigned potential at the walls.
Best John
April 11, 2022 at 10:04 amYago
SubscriberI've checked all that and still there's no reaction. I'll try to post screenshots to see if it helps find something that might be wrong
April 11, 2022 at 10:11 amYago
Subscriber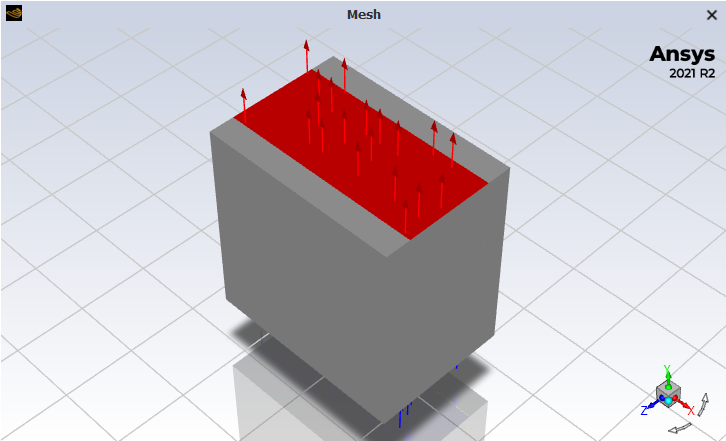
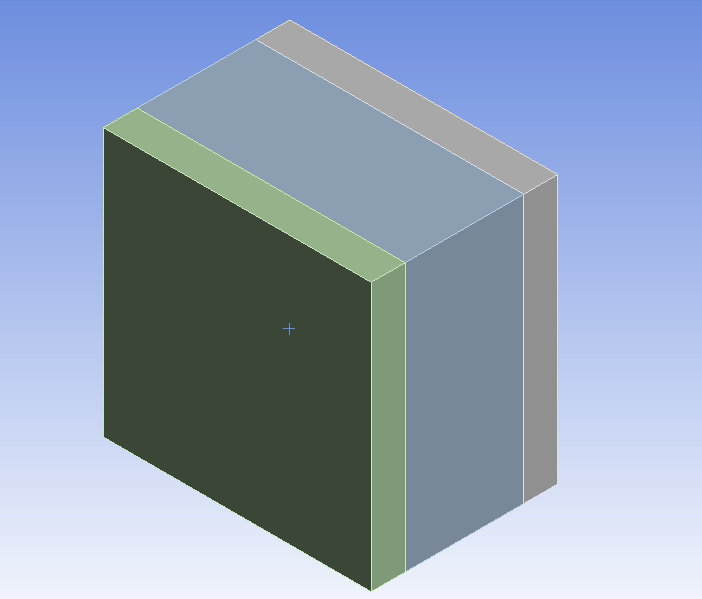 That's the geometry of the cell I have. The green and gray parts are the anode and cathode (respectively), and the blue part is the electrolyte. The bottom side is the inlet and the top side is the outlet of the electrolyte flow.
That's the geometry of the cell I have. The green and gray parts are the anode and cathode (respectively), and the blue part is the electrolyte. The bottom side is the inlet and the top side is the outlet of the electrolyte flow.
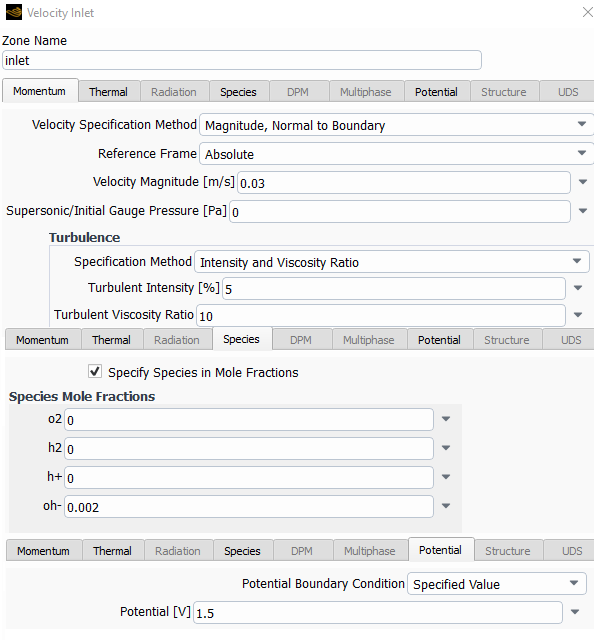
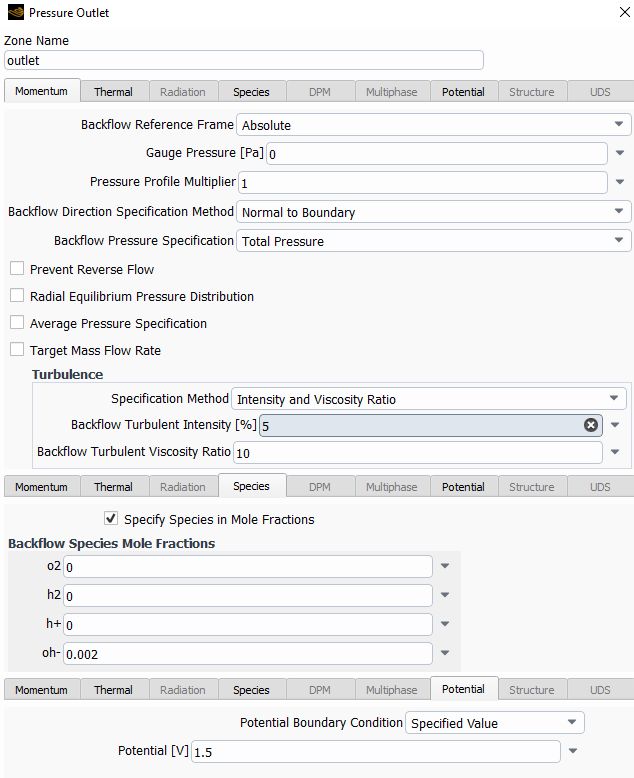
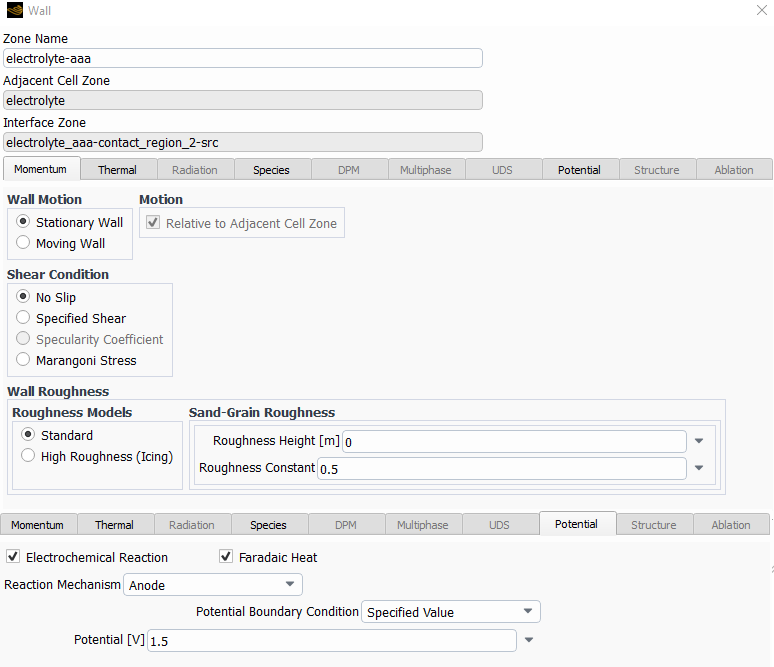
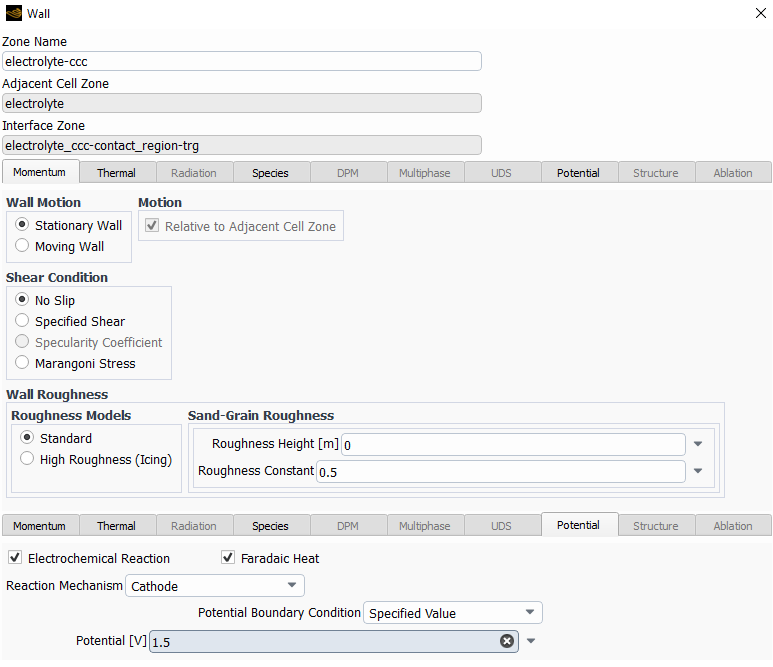
The following are the species model, with the electrochemical reaction model and the two different reactions.
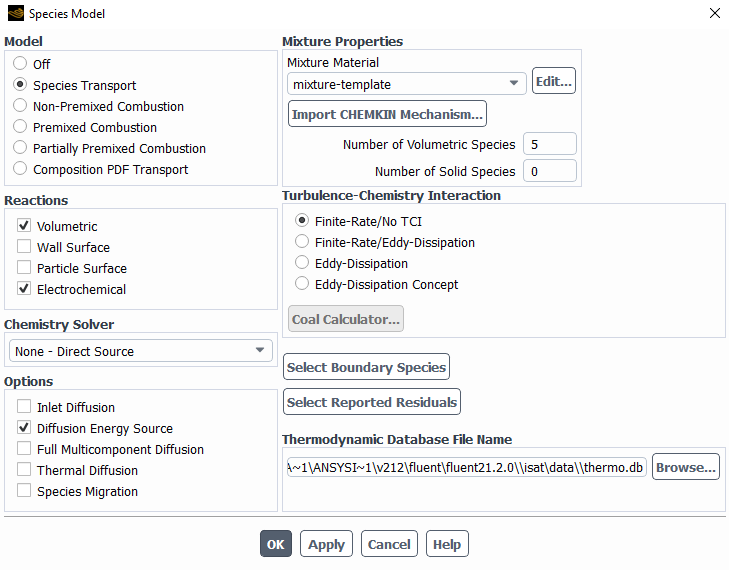
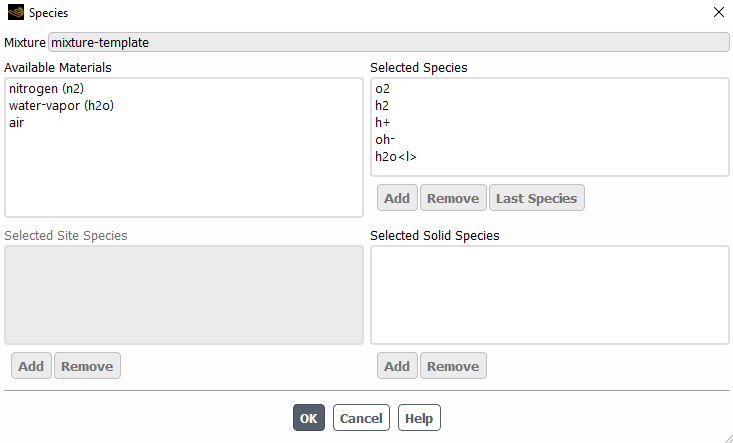
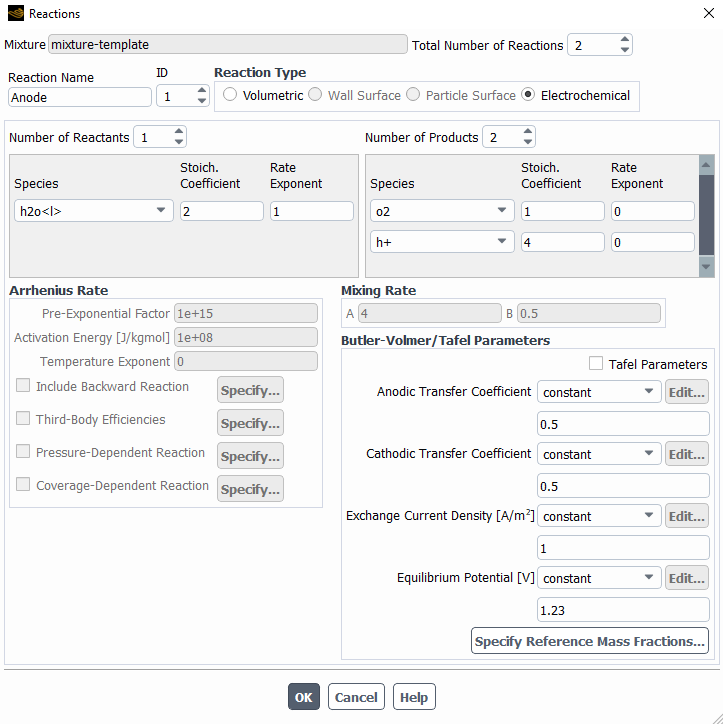
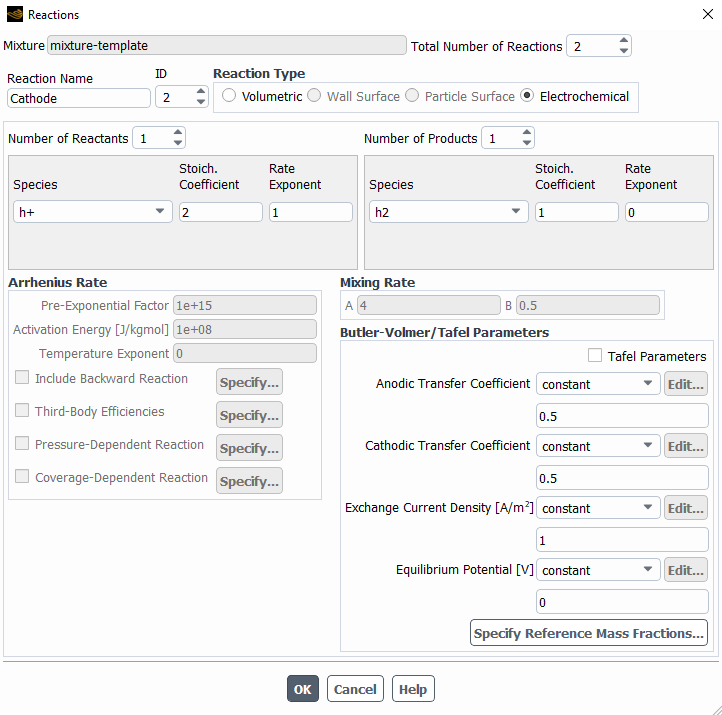
I left most of the values as default with the idea in mind to change them once I make it work.
April 11, 2022 at 10:14 amApril 11, 2022 at 10:16 amOctober 9, 2023 at 5:45 amh.mohamed_mohsin
SubscriberHi, I am wondering did you managed to get the electrochemical reaction to occur on the electrode?
October 9, 2023 at 8:11 amYago
SubscriberHi. I managed to get the reaction changing to a conformal mesh.
October 10, 2023 at 2:27 amh.mohamed_mohsin
SubscriberHi, I tried to use the shared topology to create conformal mesh, but then there is no boundry walls between the electrolyte and the electrodes. How do you specify the reactions on the wall then? I am wondering can I get your email as it would be easier to contact if you don't mind.
October 17, 2023 at 5:24 amh.mohamed_mohsin
SubscriberHi, I am still struggling to get the model to work, wondering where did you apply the electrochemical reaction to allow the model to work?
Viewing 11 reply threads- The topic ‘Water splitting electrochemical reaction’ is closed to new replies.
Innovation SpaceTrending discussionsTop Contributors-
4783
-
1565
-
1386
-
1242
-
1021
Top Rated Tags© 2026 Copyright ANSYS, Inc. All rights reserved.
Ansys does not support the usage of unauthorized Ansys software. Please visit www.ansys.com to obtain an official distribution.
-


Ansys Assistant

Welcome to Ansys Assistant!
An AI-based virtual assistant for active Ansys Academic Customers. Please login using your university issued email address.
Hey there, you are quite inquisitive! You have hit your hourly question limit. Please retry after '10' minutes. For questions, please reach out to ansyslearn@ansys.com.
RETRY