TAGGED: electrochemical-cell, electrochemistry, electrolysis, fluent, reaction
-
-
January 23, 2024 at 4:38 pm
teresa.oliveira
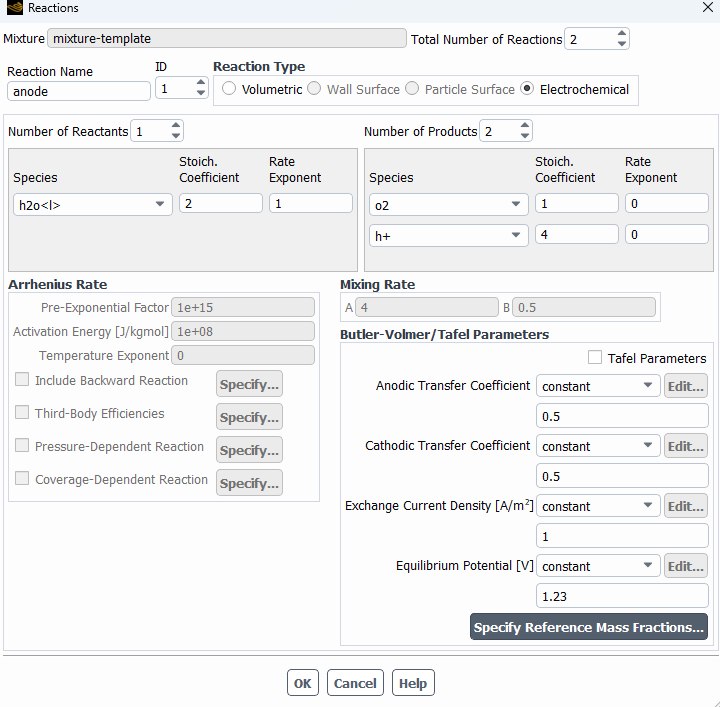
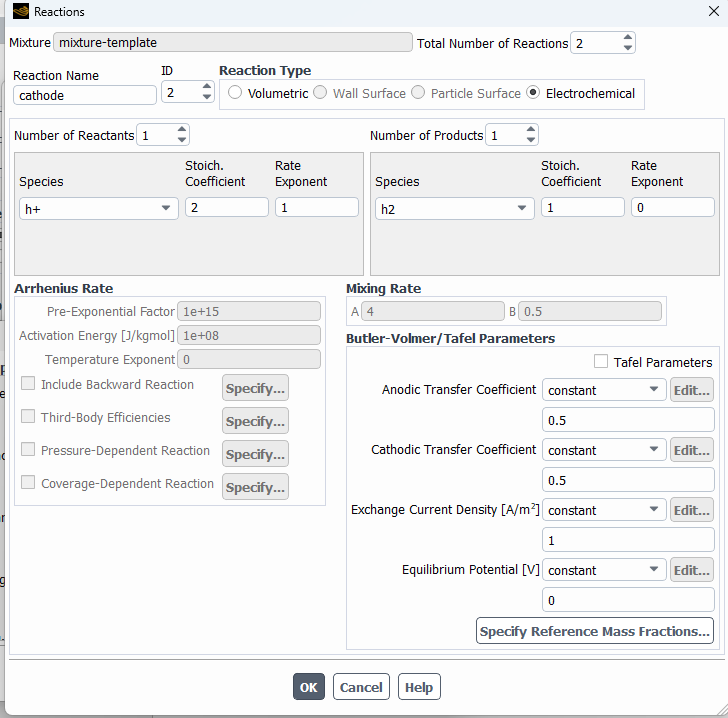
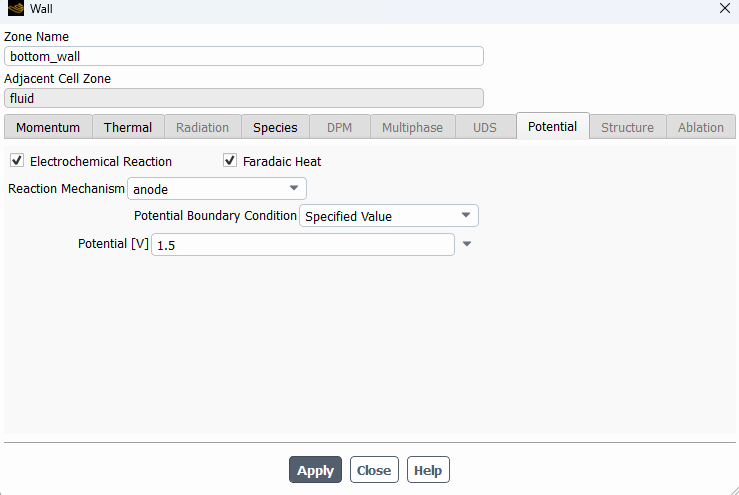
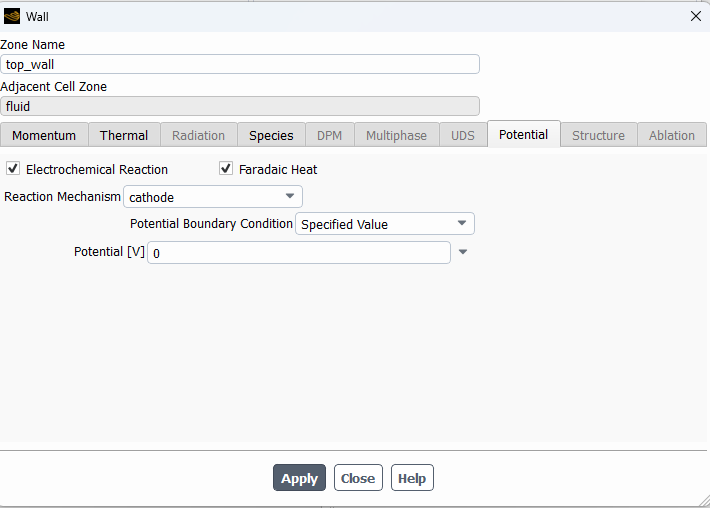
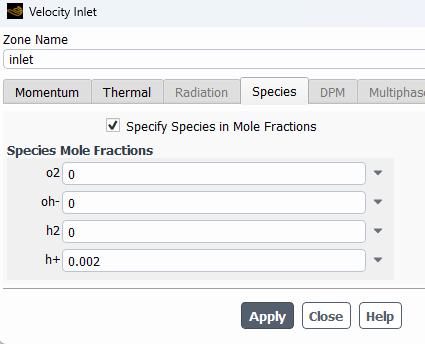
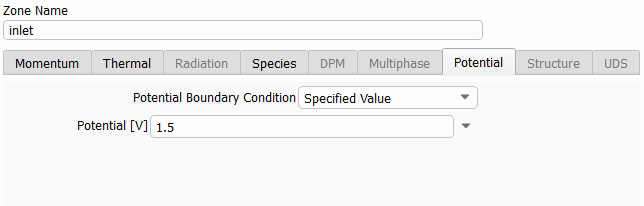
SubscriberHi! I'm modelling the electrolysis of water in an electrochemical cell and I've used the potential model with the Joule heating equation activated. I've defined electrochemical reactions at both electrodes. In the anode side I set a potential of 1.5 V, with an equilibrium potential of 1.23 V, while on the cathode side I set 0 V. I attach images with the settings used. I am not getting convergence and the oxygen isn't being formed at all. Can someone help me to figure out what I am doing wrong? Should I define the reactions or the boundary conditions in a different way? Thanks in advance
-
February 5, 2024 at 11:18 pm
John Ibrahim
Ansys EmployeeHello Teresa,
You do not have to turn on the wall reaction as you are doing now. Can you tell me more about your physical problem. For the convergence, can you try to switch to transient solver?
Best,
John
-
- The topic ‘Simulation of Electrochemical Reactions’ is closed to new replies.



-
4678
-
1565
-
1386
-
1242
-
1021

© 2025 Copyright ANSYS, Inc. All rights reserved.