TAGGED: #fluent-#ansys, ansys-cfd, cfd-combustion, chemical-reaction
-
-
April 21, 2024 at 2:49 pm
315914335
Subscriber -
April 22, 2024 at 3:47 pm
315914335
Subscriberupup -
April 28, 2024 at 8:13 am
315914335
Subscriberupup -
May 20, 2024 at 3:57 pm
Ren
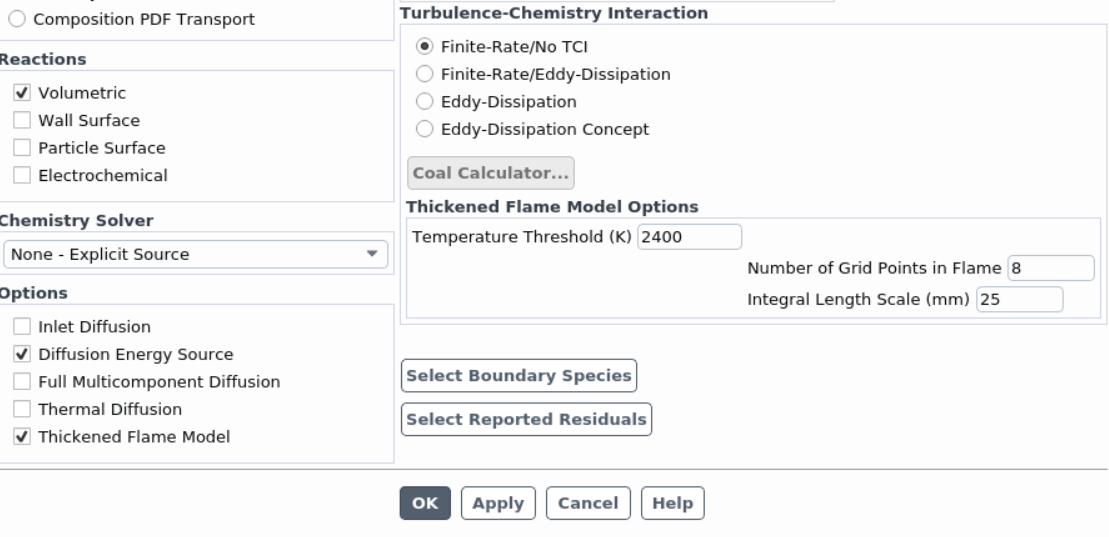
Ansys EmployeeA likely cause of the excessively high temperature is the lack of inadequate convergence of the energy equation. Please try the "Stiff Chemistry Solver" that can be selected from the dropdown list under "Chemistry Solver".
The Temperature Threshold is to save computational cost by assuming the reaction rate is zero for temperature values below the threshold.
-
May 21, 2024 at 3:37 pm
jcooper
Ansys EmployeeHi Jiacheng:
A one-step mechanism is very crude chemistry to describe a process that in reality has hundreds of chemical reactions. For an example, you can visit the UC San Diego page, where the base mechanism can be used + the heptane chemistry to approximate kerosine:
https://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html
Another way to approach fuel mixtures is to develop surrogate fuel mixtures for which the chemistry is known. This type of work can be conducted with Chemkin and is described in the paper at the link below:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8444312/
-
- The topic ‘Setting of thickened flame model in FLUENT’ is closed to new replies.



-
4939
-
1639
-
1386
-
1242
-
1021

© 2026 Copyright ANSYS, Inc. All rights reserved.