TAGGED: ansys-fluent, cfd-combustion, specific-heat
-
-
December 13, 2025 at 11:50 am
imjsh12
SubscriberI'm trying to figure out the actual mechanism of chemical species transfer combustion [H2/O2].
Chemkin's "opposed-flow_flame/h2_air/chem.inp" was successfully applied, but combustion did not occur.
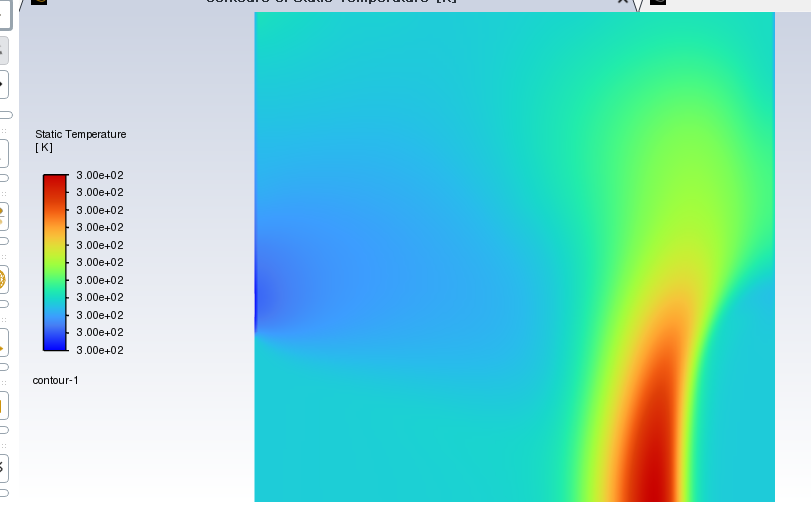
This is simply a mixture of different substances in the air!
[Main flow direction -> , <-]
There are no errors, so why doesn't combustion occur?
Current
Chemkin file -> Non-mixed flame: Success
Chemical species transfer -> Default file "Methane-Air": Success
Chemkin file -> Chemical species transfer: FailureReference link: https://ansyshelp.ansys.com/public/account/secured?returnurl=/Views/Secured/corp/v242/en/flu_tg/flu_tg_magnus.html
-
December 13, 2025 at 11:52 am
-
December 13, 2025 at 11:56 am
imjsh12
Subscriberand chemkin .inp file
-------------------
ELEMENTS
H O N
END
SPECIES
H2 H O2 O OH HO2 H2O2 H2O N2
END
REACTIONS
H+O2+M=HO2+M 3.61E17 -0.72 0. !DIXON-LEWIS
H2O/18.6/ H2/2.86/
H+H+M=H2+M 1.0E18 -1.0 0.
H+H+H2=H2+H2 9.2E16 -0.6 0.
H+H+H2O=H2+H2O 6.0E19 -1.25 0.
H+OH+M=H2O+M 1.6E22 -2.0 0. !D-L
H2O/5/
H+O+M=OH+M 6.2E16 -0.6 0. !D-L
H2O/5/
O+O+M=O2+M 1.89E13 0.0 -1788. !NBS
H2O2+M=OH+OH+M 1.3E17 0.0 45500.
H2+O2=2OH 1.7E13 0.0 47780.
OH+H2=H2O+H 1.17E9 1.3 3626. !D-L$W
O+OH=O2+H 3.61E14 -0.5 0. !JAM 1986
O+H2=OH+H 5.06E4 2.67 6290. !KLEMM,ET AL 1986
OH+HO2=H2O+O2 7.5E12 0.0 0.0 !D-L
H+HO2=2OH 1.4E14 0.0 1073. !D-L
O+HO2=O2+OH 1.4E13 0.0 1073. !D-L
2OH=O+H2O 6.0E+8 1.3 0. !COHEN-WEST.
H+HO2=H2+O2 1.25E13 0.0 0. !D-L
HO2+HO2=H2O2+O2 2.0E12 0.0 0.
H2O2+H=HO2+H2 1.6E12 0.0 3800.
H2O2+OH=H2O+HO2 1.0E13 0.0 1800.
END------------------------
-
December 13, 2025 at 5:23 pm
imjsh12
SubscriberI did it! I did it myself!
It was just a simple solution.
Physucs -> Species Transfer -> chemkin import -> turvulence ~ : check "Eddy-dissipation"! -> chemistry solver : check "Relax to" ~!I don't understand how the difference between "non ~" and "Relax to ~" modes in the chemistry solver options determines the success or failure of combustion.
-
December 15, 2025 at 1:27 pm
Ren
Ansys EmployeeSome combustion models require numerical ignition to initiate combustion and some models do not.
-
December 15, 2025 at 5:36 pm
sam.whitman
SubscriberYes, to add to what Ren posted, H2 in air at STP should not autoignite at 300K. Relaxing to chemical equilibrium can bypass (or substitute for) the ignition step, as you are not actually solving the finite-rate kinetics with this method. Please refer to the Theory Guide to read more about this approach.
Because of the above, if you are seeking to incorporate the full kinetic mechanism in your solution you should switch to a different solver and/or combustion method after achieving this initial combustion - you can read more about the available options in the Theory Guide.
-
- You must be logged in to reply to this topic.



-
4602
-
1510
-
1386
-
1209
-
1021

© 2025 Copyright ANSYS, Inc. All rights reserved.