-
-
June 9, 2021 at 8:27 am
Propanotriol
SubscriberDear all,
I am trying to model a furnace where different reactants react with oxygen (combustion). The reactions are not at all complex. I am using a CHEMKIN mechanism:
ELEMENTS C O S H N
END
SPECIES
COS O2 CO2 SO2 H2S H2O C2H6S CO C4H8O S N2
END
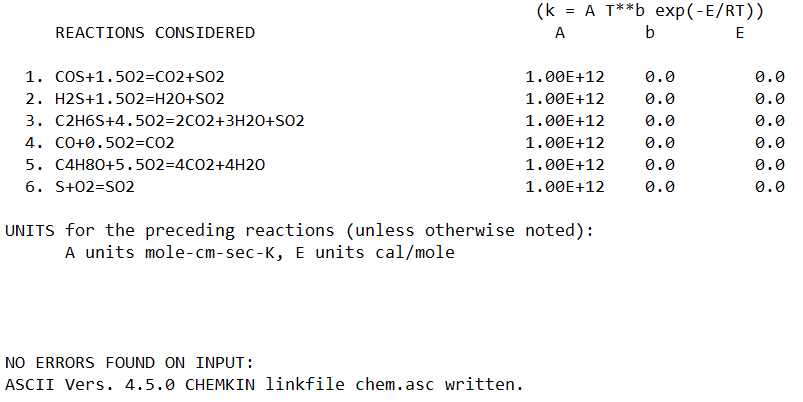
REACTIONS
COS+1.5O2=CO2+SO2 1E6 0 0
H2S+1.5O2=H2O+SO2 1E6 0 0
C2H6S+4.5O2=2CO2+3H2O+SO2 1E6 0 0
CO+0.5O2=CO2 1E6 0 0
C4H8O+5.5O2=4CO2+4H2O 1E6 0 0
S+O2=SO2 1E6 0 0
END
Since I do not know the Ahrrenius coefficients for the kinetics, I decided to model the reactions as instantaneous and fast (0 activation energy and high value for A).
The thermodynamic database is correct and constructed from the "Third Millennium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with Updates from Active Thermochemical Tables" by Burcat and Ruscic.
All the set-up is correct and as it should be. The simulation evolves correctly, no divergence at all and convergence of different controls such as mass conservation, energy etc. are also good.
HOWEVER, the reaction involving C2H6S and C4H8O do not work. This is, they are not consumed at all. And yes, there is enough oxygen in the system. I don't understand why all the other 4 mechanisms do work and the reactants are correctly consumed (inexistent at the outlet) while for these two, I recover exactly the same ammount that I introduce into the system.
June 14, 2021 at 2:41 amRahul Kumar
Ansys EmployeeHello,
Please make sure the stoic. coefficients and the rate exponents are defined right. These are the parameters that would affect the consumption of the species.
June 14, 2021 at 9:23 amJune 14, 2021 at 1:48 pmRahul Kumar
Ansys EmployeeCan you try varying the values for 3 and 5?
June 14, 2021 at 4:47 pmPropanotriol
SubscriberI did, I increased k several orders of magnitude for reaction 3 and 5 and still not working.
Viewing 4 reply threads- The topic ‘[FLUENT] Species Model: CHEMKIN Mechanism’ is closed to new replies.
Ansys Innovation SpaceTrending discussionsTop Contributors-
3597
-
1243
-
1092
-
1068
-
953
Top Rated Tags© 2025 Copyright ANSYS, Inc. All rights reserved.
Ansys does not support the usage of unauthorized Ansys software. Please visit www.ansys.com to obtain an official distribution.
-


Ansys Assistant

Welcome to Ansys Assistant!
An AI-based virtual assistant for active Ansys Academic Customers. Please login using your university issued email address.
Hey there, you are quite inquisitive! You have hit your hourly question limit. Please retry after '10' minutes. For questions, please reach out to ansyslearn@ansys.com.
RETRY