-
-
October 31, 2023 at 9:53 am
Erik Gerlings
SubscriberHi,
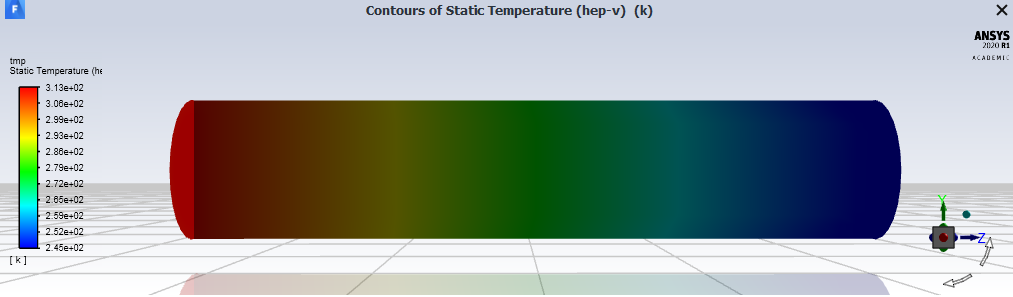
I am trying to make a multiphase model which involves the condensation of n-heptane.
An issue that I run into in this case is that even for the simplest mass transfer mechanism (constant rate), the temperature of the fluid decreases. My from fluid is the liquid heptane and to liquid is heptane vapor.
Could someone please explain why the temperature decreases while for condensation the temperature should increase? And how can I resolve this problem?
Kind regards,
Erik
-
October 31, 2023 at 3:43 pm
Rob
Forum ModeratorIf the gas is condensing, what density model did you use? Ie where does the extra volume come from if the system is sealed?
-
November 1, 2023 at 1:04 am
Erik Gerlings
SubscriberThank you for your fast response.
It is an open system, the inflow is 3 kg/s (left) and the outlet is a pressure outlet (right).
-
November 1, 2023 at 1:42 am
Erik Gerlings
SubscriberI found that the standard state enthalpy for heptane vapour is -1.878e+08 j/kmol which causes the temperature decrease. Thanks for you attention.
-
- The topic ‘Condensation with decreasing temperature’ is closed to new replies.



-
4663
-
1540
-
1386
-
1230
-
1021

© 2025 Copyright ANSYS, Inc. All rights reserved.