-
-
March 10, 2024 at 1:12 pm
Diri Li
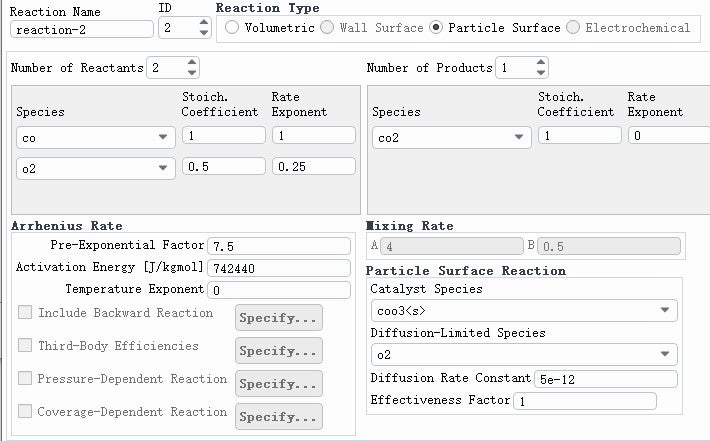
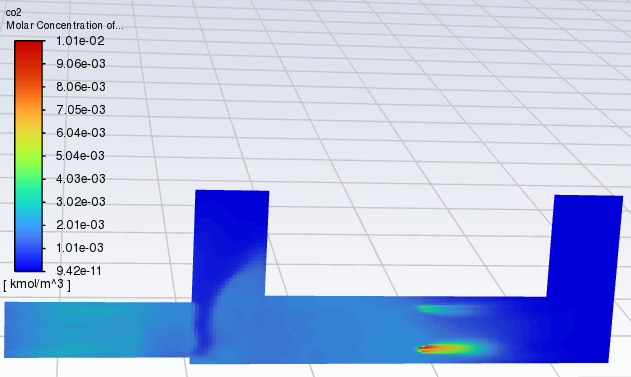
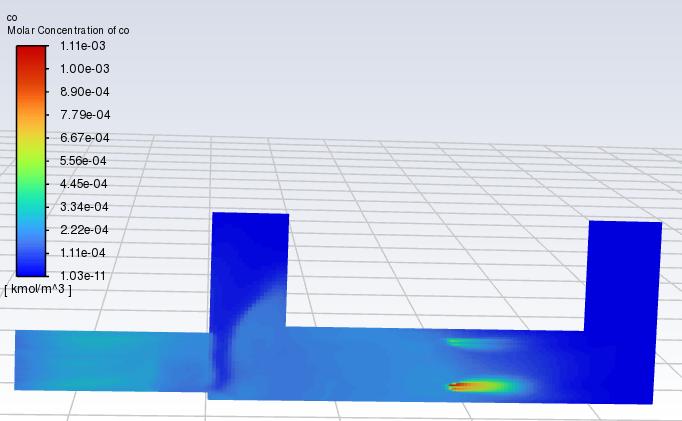
SubscriberI tried simulating an oxidation reaction, but the results are off. The amount of CO2 produced and the decrease in CO don't match the expected values based on the reaction equation. It seems like too much CO2 is being generated, which shouldn't be possible according to the stoichiometry of the reaction. Do you think there might be an issue with my simulation settings? I've included my simulation results and reaction parameters below. Your feedback would be greatly appreciated. Thanks a lot!
-
March 11, 2024 at 12:07 pm
Rob
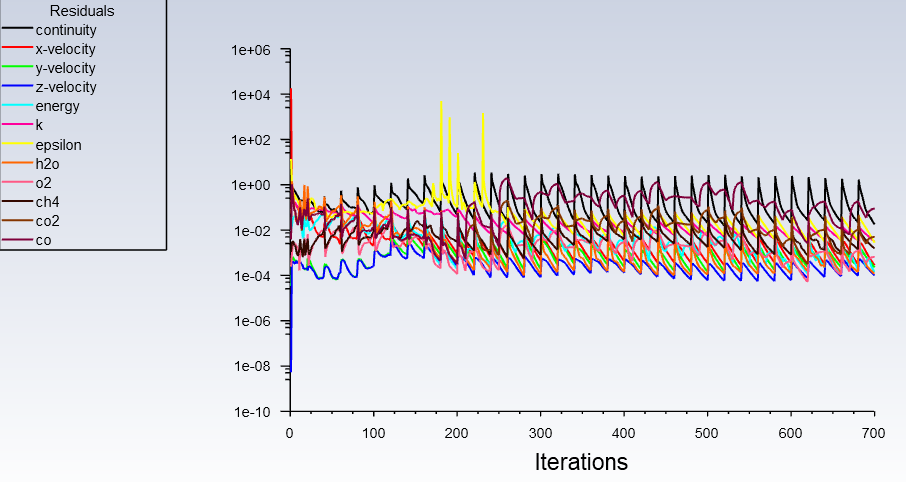
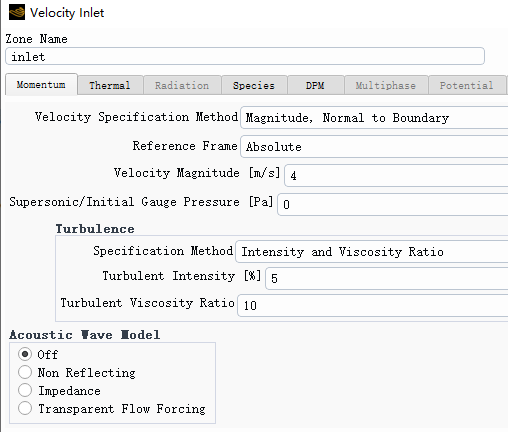
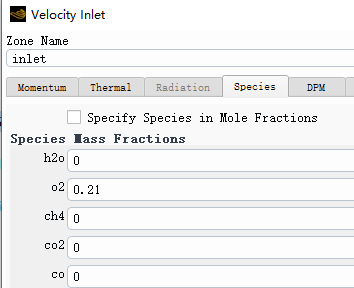
Forum ModeratorPlease can you post images of the mesh, residuals and boundary conditions. Also, repost the above images but with node values off (contour panel setting).
-
March 12, 2024 at 6:04 am
-
March 12, 2024 at 10:11 am
Rob
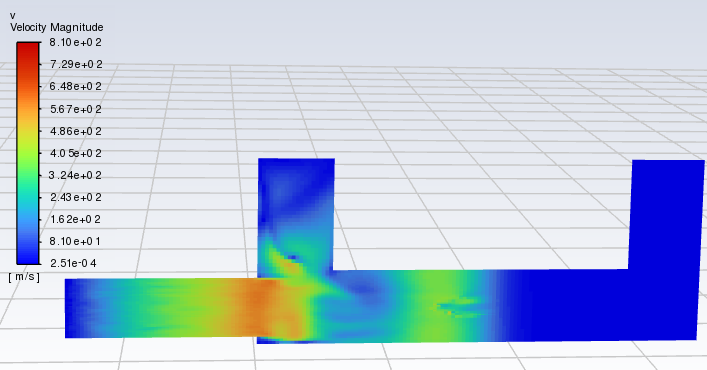
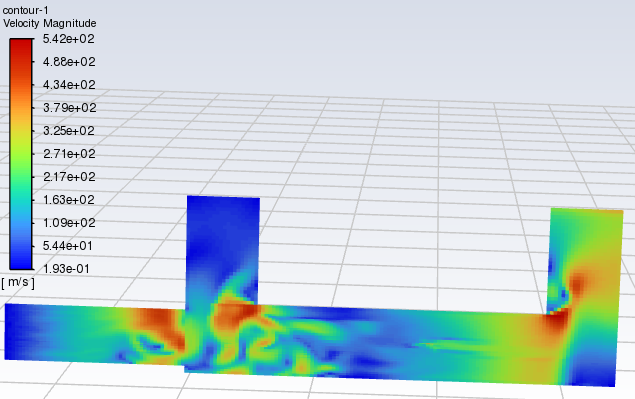
Forum ModeratorThanks. Skew & resolution look OK in the bulk flow. But if that residual plot is transient you've only done 30 timesteps? Can you plot velocity please? You need to understand the whole flowfield (not just monitors or species) to figure out what's going on.
-
March 13, 2024 at 5:31 am
-
March 13, 2024 at 10:16 am
Rob
Forum ModeratorShould a combustor really be running at 5-800m/s ?
-
March 13, 2024 at 11:57 am
Diri Li
SubscriberI simulated an explosion with high temperature and pressure airflow.
-
March 13, 2024 at 3:17 pm
Rob
Forum ModeratorRoughly how long does it take the flow to cross a single cell at the speeds you're seeing?
-
March 15, 2024 at 3:11 am
Diri Li
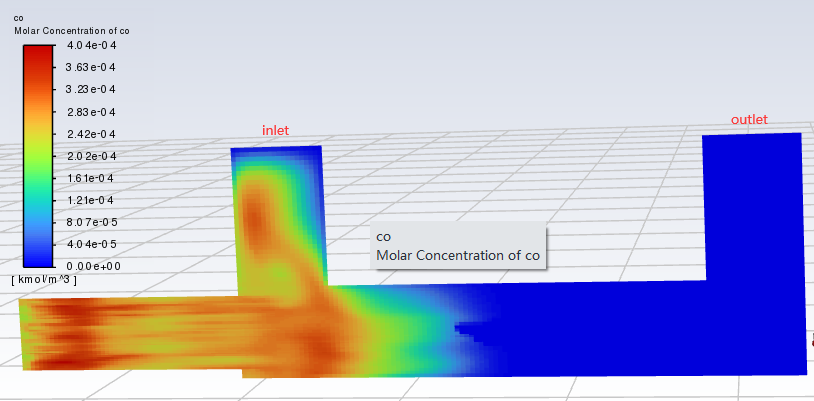
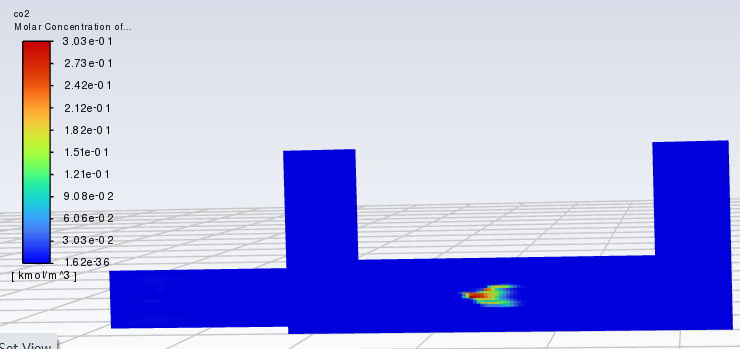
SubscriberThe time to pass through a single cell is approximately between 0.00001 and 0.002 s. I calculated a model without setting the reaction of CO+0.5O2=CO2, and the results showed that after contact with particles, the concentrations of CO and CO2 increased(As shown in the figure). Why is this? Does Fluent have a built-in reaction for burning particles? For example, C+O2=CO2? Thank you for any help.
-
March 15, 2024 at 11:24 am
Rob
Forum ModeratorNo, but if convergence is poor it's possible the solver has added species in as it begins to fail. What time step are you using?
-
March 15, 2024 at 12:10 pm
Diri Li
SubscriberI set a time step of 0.0001s.
-
March 15, 2024 at 2:37 pm
Rob
Forum ModeratorOK, and at the flow rate seen how long would it take to flush the initial condition from the domain?
-
March 15, 2024 at 3:31 pm
Diri Li
SubscriberI'm sorry to say that I only calculated 0.01s, and did not vent the gas completely. I haven't calculated such a long time.
I'll give it a try and get back to you with a definite answer.
I'm very curious to see how I can control the reaction I've set, it'll be very interesting!
I'm very grateful for your patience!! -
March 15, 2024 at 3:49 pm
Rob
Forum ModeratorWhich means you're possibly looking at a remnant of the initial condition. In transient models you have a flow time scale (related to velocity and cell size, ie a very small number) and a domain timescale (related to overall volume and volume change rate, often many minutes). If you're not modelling too many times the latter value you may be looking at the result of the short run and it's dominated by the initial condition.
-
March 15, 2024 at 4:07 pm
Diri Li
SubscriberI understand!
I need time to try and verify it. If I still have questions, will I be able to continue to consult you below this post?
Thank you for your answer! -
March 15, 2024 at 4:24 pm
Rob
Forum ModeratorFor a continuation post here (ideally) for new topics please post in a new thread. My presence is workload related - so I'm not always here!
-
- The topic ‘chemical reaction’ is closed to new replies.



-
4833
-
1587
-
1386
-
1242
-
1021

© 2026 Copyright ANSYS, Inc. All rights reserved.