Ansys Learning Forum › Forums › Discuss Simulation › Fluids › Particle surface reaction › Reply To: Particle surface reaction
HI Diri:
None of what you describe seems unphysical, CO2 is a product and will evolve from the particle surface as particles come into contact with O2. N2 molar concentration will go down in the areas where CO2 is produced (because the mass fractions all have to add to 1).
What you can get is an overproduction of CO2 due to numerics. This is related to the fact that particle solution is staggered from the fluids solution, so particle reactions happen on an outdated fluid solution field. If you are getting this type of overshoot, the oxygen will in the areas of high CO2 will be at or near 0.
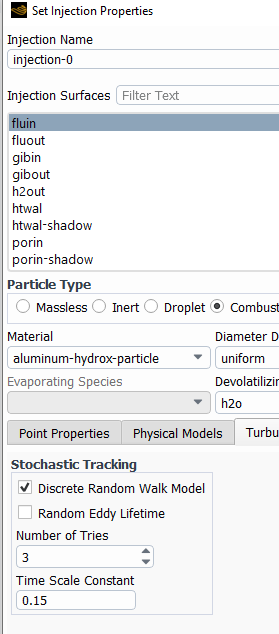
The remedy for this is to inject particle more frequently (every 2 fluid calculations), and also inhject more particles (by turning on turbulent dispersion). This will calculate more trahectories and the particle sources will be spread over a larger area. Turbulent dispersion is activated under each injection. The number of tries is the multiplier for the number of trajectories.
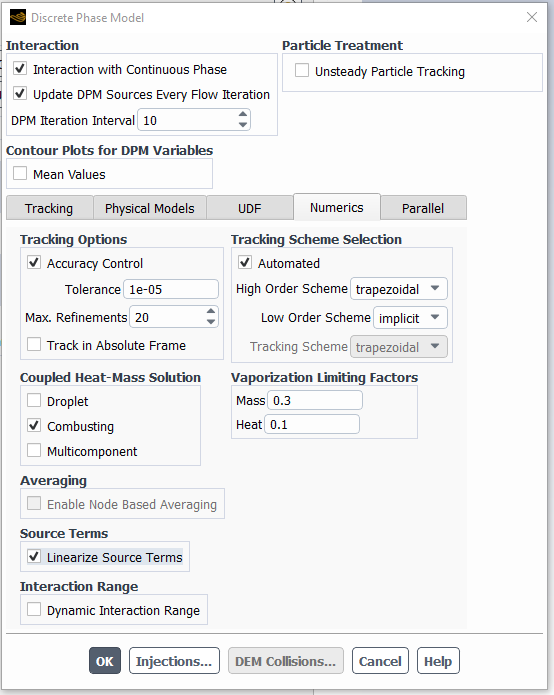
Other things to try are to turn on particle source linearization Under Discrete phase model Numerics tab:
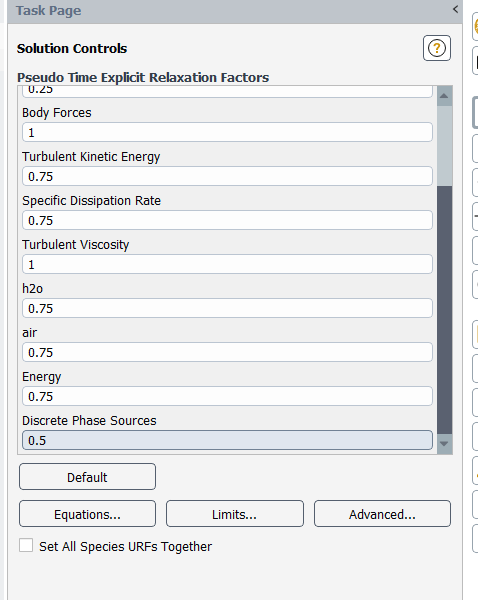
...and underrelax the particle sources too - you can set this parameter as low as 0.1
(I would also ensure that species URFs and discretizations are set together, both on this form and under Methods. This ensures that all species equations get the same numerical treatment.)
I hope this helps.
Best Regards,
Judy